At least 460 cases of acute hepatitis of unknown origin in children have been reported worldwide, including 12 deaths and dozens of people requiring liver transplantation.
Most children present with early gastrointestinal symptoms, which later develop jaundice and, in some cases, acute liver failure. To the confusion of scientists, the hepatitis A, B, C, D and E viruses were not found in these children. The current figures may be just the tip of the iceberg, as many countries are just beginning to ramp up surveillance for this unexplained childhood case of acute hepatitis.
The U.S. Centers for Disease Control and Prevention (CDC) held a telephone/website meeting for clinicians on May 19, local time, titled "Testing and Clinical Recommendations for Acute Hepatitis Adenovirus in Children of Unknown Cause".
Although the CDC issued a disclaimer, the content of the exchange still drew criticism from the clinical community and virologists. The main criticisms include: continuing to discuss adenovirus infection as the first cause of acute hepatitis of unknown cause in children, introducing adenovirus at length, and giving guidance on adenovirus testing, but there is absolutely no recommendation for serum COVID-19 antibody testing in possible cases . At the same time, the use of the antiviral drug cidofovir was mentioned in the case sharing when the acute hepatitis caused by adenovirus could not be determined.
In healthy people, adenovirus adeno-self-limiting disease can cause respiratory, gastrointestinal, or conjunctival inflammation. Severe conditions that can lead to acute liver failure and even death have never been seen in people with normal immune systems. Adenoviruses can cause liver failure only in people with severely compromised immune systems, such as those receiving organ transplants and chemotherapy.
Isabella Eckerle, head of the Center for Emerging Infectious Diseases at the University of Geneva, Switzerland, questioned that adenovirus was not detected in liver biopsies of children with acute hepatitis. If it is caused by adenovirus, the liver should have adenovirus-related pathology. The virus replicated in the liver of the child should be found everywhere: in plasma, serum, biopsies, not only in whole blood in most children.
In fact, the adenovirus Ct values of the children are relatively high, which means that the viral load is relatively low, which is why only some children can perform adenovirus gene fragment detection for adenovirus, and it is found to be adenovirus type 41. None of the cases had a viral load high enough for adenovirus genome-wide testing.
The first speaker at the CDC symposium was L. Amanda Ingram, epidemiologist and director of infectious diseases at the Alabama Department of Public Health. He described 12 cases of acute hepatitis of unknown origin in children in Alabama. During the teaching session, his question raised doubts.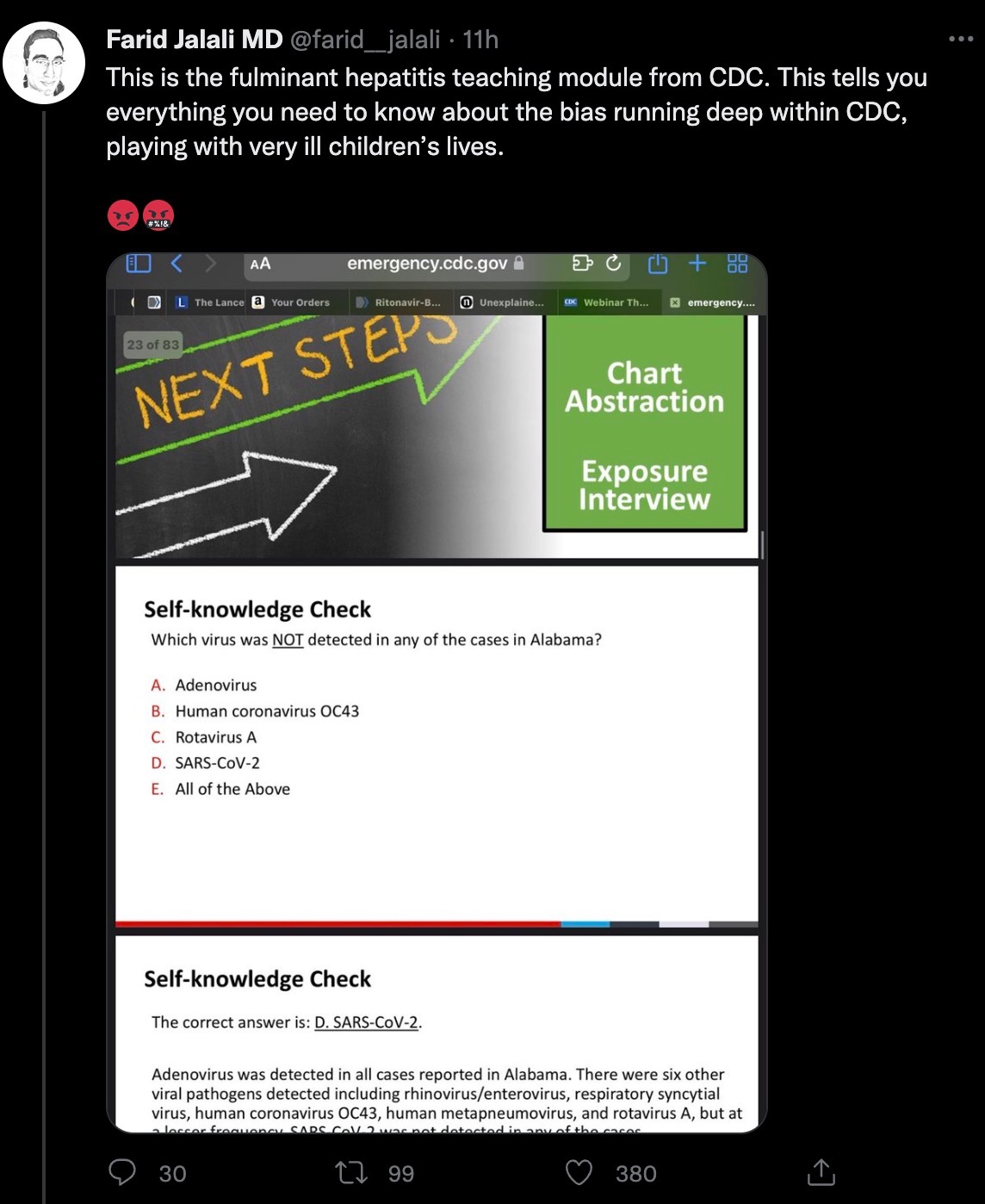 The Alabama health official asked the question: Which of the following viruses has not been detected in Alabama cases? The 5 options are: adenovirus, coronavirus OC43, rotavirus, new coronavirus, all of the above. The answer is D, the new coronavirus.
The Alabama health official asked the question: Which of the following viruses has not been detected in Alabama cases? The 5 options are: adenovirus, coronavirus OC43, rotavirus, new coronavirus, all of the above. The answer is D, the new coronavirus.
However, the Alabama child only had a positive nucleic acid test on a nasopharyngeal swab when he was admitted to the hospital. In the absence of a serological new crown antibody test, the possibility of previous infection with the new coronavirus cannot be ruled out. In this regard, Farid Jalali, a California physician, Ph.D., gastroenterologist and hepatologist at the University of California, Irvine, said, "This is the teaching link of the US CDC on acute hepatitis of unknown cause in children. This is to tell you: there is a serious problem within the US CDC. They are making fun of the lives of seriously ill children."
In fact, the latest article in The Lancet Gastroenterology & Hepatology, an international authoritative academic journal, proposes that acute hepatitis of unknown cause in children may be related to the superantigen mechanism after previous infection with the new coronavirus .
The above article stated that infection of children with the new coronavirus can lead to the formation of a virus reservoir in the body. Specifically, the persistent presence of 2019-nCoV in the gastrointestinal tract of children can lead to the repeated release of viral proteins in intestinal epithelial cells, resulting in immune activation. This repeated immune activation may be mediated by a superantigen motif in the spike protein of 2019-nCoV, which is similar to staphylococcal enterotoxin B and triggers broad and nonspecific T cell activation. This superantigen-mediated activation of immune cells has been suggested to be the mechanism behind Multisystem Inflammatory Syndrome in Children (MIS-C).
The so-called super antigen (SAg) is a kind of substance that can activate a large number of T cell clones and generate a strong immune response with only a very low concentration (≤10-9 times M). Compared with ordinary antigens, superantigens do not require conventional intracellular antigen presentation and have no MHC restriction.
According to the different activated cells, superantigens can be divided into T cell superantigens and B cell superantigens, and according to their different sources, T and B cell superantigens can be divided into endogenous (viral) antigens and exogenous (viral) superantigens. bacterial type) superantigens.
Acute hepatitis has also been previously reported in children with multisystem inflammatory syndrome, but coinfection with other viruses has not been investigated. The researchers analyzed that the recent acute hepatitis of unknown cause in children may have been infected with the new coronavirus first, and the children were infected with adenovirus after the virus reservoir appeared in the intestine.
Specifically, as shown in the following figure: Schematic diagram of the pathology of adenovirus (AdV)-enhanced SARS-CoV-2 superantigen-mediated severe acute hepatitis.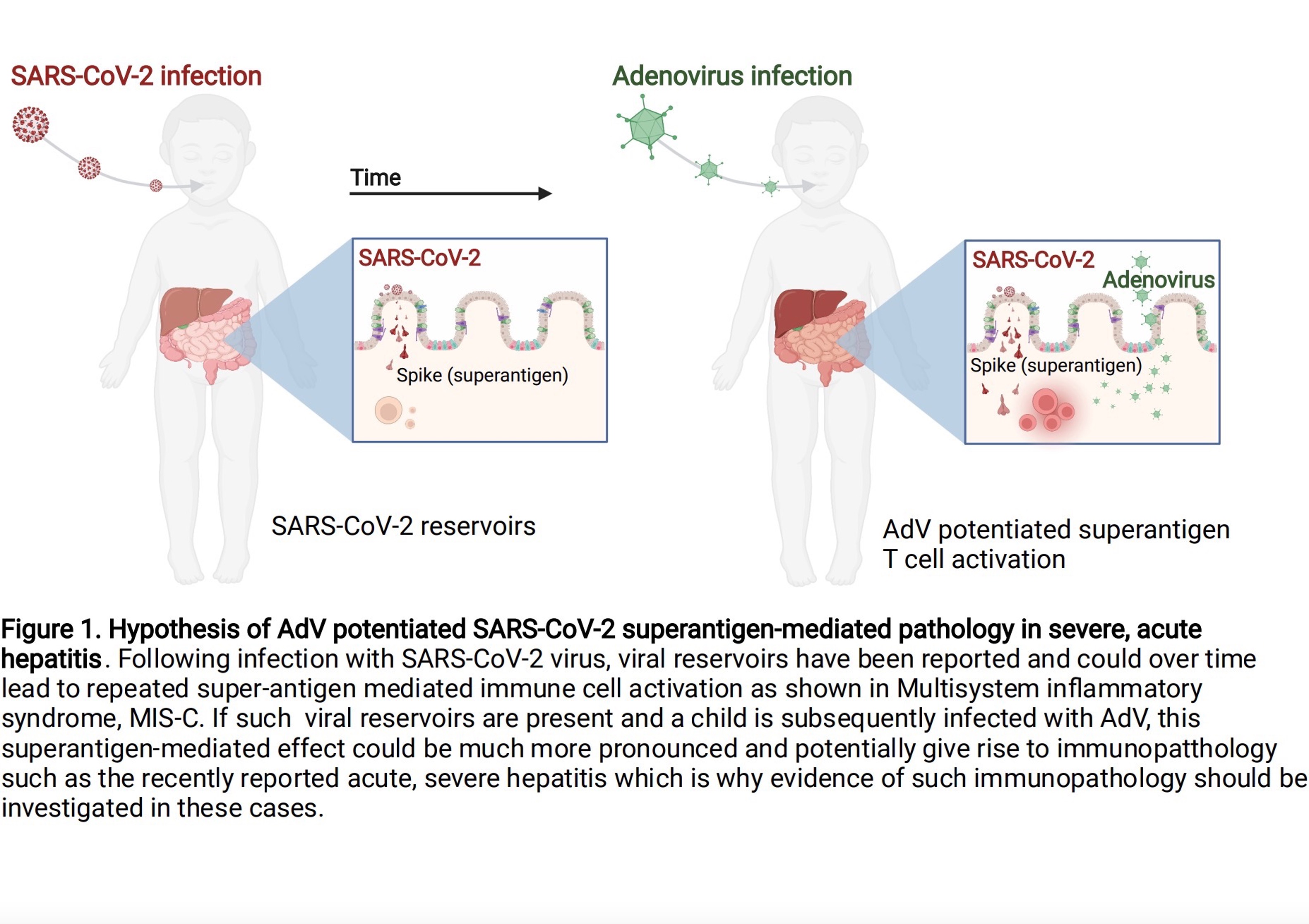
However, in the CDC sharing meeting, there was not much content about the investigation of serum new crown antibodies in children, and it was impossible to discuss the superantigen mechanism after new crown infection. Deepti Gurdasani, an epidemiologist at Queen Mary University of London, questioned, "I have to say that I was very surprised to see the recent CDC report on fulminant hepatitis in children. There has been so much discussion on the adenovirus issue, and the new crown serum has been mentioned by the way. Scientific investigation (but not yet completed). None of the liver biopsies found adenovirus, but the children were treated with antiviral drugs.” In a CDC sharing session, Elizabeth A. Moulton, an assistant professor at Texas Children's Hospital, mentioned the use of the antiviral drug cidofovir on some American children.
In a CDC sharing session, Elizabeth A. Moulton, an assistant professor at Texas Children's Hospital, mentioned the use of the antiviral drug cidofovir on some American children.
Available data show that cidofovir is an open-loop nucleotide analog and is a new drug against cytomegalovirus (CMV). For other herpes viruses, such as herpes simplex virus type I and type II (HSV-I, HSV-II), varicella-herpes virus (VZV), Epstein-Barr virus, herpes virus type 6 (HHV-6), adenovirus and human Papillomatosis (HPV) is very active.
"From my point of view as a clinical virologist, there are many arguments against why adenoviruses cause acute hepatitis," says Eckerle, which has important implications for treatment. Why? This means that whether antiviral therapy or immunosuppressive therapy (such as cortisol) should be used can have very different effects on a patient's clinical outcome.
If the new coronavirus superantigen is considered, hormonal immunosuppressive therapy should be used. In fact, the latest May issue of JPGN REPORTS, a journal of paediatric gastroenterology and hepatology, reported a rare case of a healthy girl who contracted COVID-19 with mild symptoms but then developed acute liver failure. Doctors at Cincinnati Children's Hospital Medical Center and the University of Cincinnati School of Medicine use intravenous methylprednisolone to avoid liver transplants.
Even taking a step back and assuming adenovirus plays a role here, isn't it more likely that it's still an inflammatory response after infection, given the absence of adenovirus in the liver, Gurdasani said? Shouldn't treatment target this (with immunosuppression)?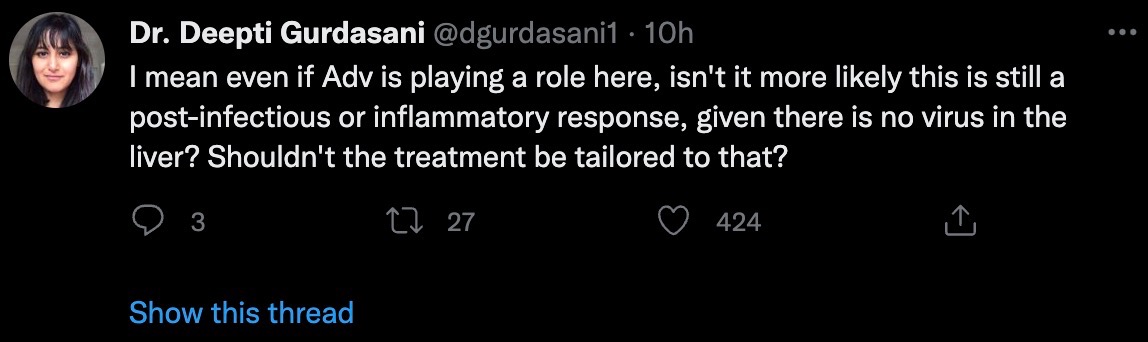
"If the CDC doesn't want to provide us with serological data on SARS-CoV-2 antibodies in children under these circumstances, that's fine," Jalali said, "but please stop promoting 'adenovirus hepatitis' + cidofovir as a treatment False assumption. This is malfeasance.”
Most children present with early gastrointestinal symptoms, which later develop jaundice and, in some cases, acute liver failure. To the confusion of scientists, the hepatitis A, B, C, D and E viruses were not found in these children. The current figures may be just the tip of the iceberg, as many countries are just beginning to ramp up surveillance for this unexplained childhood case of acute hepatitis.
The U.S. Centers for Disease Control and Prevention (CDC) held a telephone/website meeting for clinicians on May 19, local time, titled "Testing and Clinical Recommendations for Acute Hepatitis Adenovirus in Children of Unknown Cause".
Although the CDC issued a disclaimer, the content of the exchange still drew criticism from the clinical community and virologists. The main criticisms include: continuing to discuss adenovirus infection as the first cause of acute hepatitis of unknown cause in children, introducing adenovirus at length, and giving guidance on adenovirus testing, but there is absolutely no recommendation for serum COVID-19 antibody testing in possible cases . At the same time, the use of the antiviral drug cidofovir was mentioned in the case sharing when the acute hepatitis caused by adenovirus could not be determined.
In healthy people, adenovirus adeno-self-limiting disease can cause respiratory, gastrointestinal, or conjunctival inflammation. Severe conditions that can lead to acute liver failure and even death have never been seen in people with normal immune systems. Adenoviruses can cause liver failure only in people with severely compromised immune systems, such as those receiving organ transplants and chemotherapy.
Isabella Eckerle, head of the Center for Emerging Infectious Diseases at the University of Geneva, Switzerland, questioned that adenovirus was not detected in liver biopsies of children with acute hepatitis. If it is caused by adenovirus, the liver should have adenovirus-related pathology. The virus replicated in the liver of the child should be found everywhere: in plasma, serum, biopsies, not only in whole blood in most children.
In fact, the adenovirus Ct values of the children are relatively high, which means that the viral load is relatively low, which is why only some children can perform adenovirus gene fragment detection for adenovirus, and it is found to be adenovirus type 41. None of the cases had a viral load high enough for adenovirus genome-wide testing.
The first speaker at the CDC symposium was L. Amanda Ingram, epidemiologist and director of infectious diseases at the Alabama Department of Public Health. He described 12 cases of acute hepatitis of unknown origin in children in Alabama. During the teaching session, his question raised doubts.
 The Alabama health official asked the question: Which of the following viruses has not been detected in Alabama cases? The 5 options are: adenovirus, coronavirus OC43, rotavirus, new coronavirus, all of the above. The answer is D, the new coronavirus.
The Alabama health official asked the question: Which of the following viruses has not been detected in Alabama cases? The 5 options are: adenovirus, coronavirus OC43, rotavirus, new coronavirus, all of the above. The answer is D, the new coronavirus.However, the Alabama child only had a positive nucleic acid test on a nasopharyngeal swab when he was admitted to the hospital. In the absence of a serological new crown antibody test, the possibility of previous infection with the new coronavirus cannot be ruled out. In this regard, Farid Jalali, a California physician, Ph.D., gastroenterologist and hepatologist at the University of California, Irvine, said, "This is the teaching link of the US CDC on acute hepatitis of unknown cause in children. This is to tell you: there is a serious problem within the US CDC. They are making fun of the lives of seriously ill children."
In fact, the latest article in The Lancet Gastroenterology & Hepatology, an international authoritative academic journal, proposes that acute hepatitis of unknown cause in children may be related to the superantigen mechanism after previous infection with the new coronavirus .
The above article stated that infection of children with the new coronavirus can lead to the formation of a virus reservoir in the body. Specifically, the persistent presence of 2019-nCoV in the gastrointestinal tract of children can lead to the repeated release of viral proteins in intestinal epithelial cells, resulting in immune activation. This repeated immune activation may be mediated by a superantigen motif in the spike protein of 2019-nCoV, which is similar to staphylococcal enterotoxin B and triggers broad and nonspecific T cell activation. This superantigen-mediated activation of immune cells has been suggested to be the mechanism behind Multisystem Inflammatory Syndrome in Children (MIS-C).
The so-called super antigen (SAg) is a kind of substance that can activate a large number of T cell clones and generate a strong immune response with only a very low concentration (≤10-9 times M). Compared with ordinary antigens, superantigens do not require conventional intracellular antigen presentation and have no MHC restriction.
According to the different activated cells, superantigens can be divided into T cell superantigens and B cell superantigens, and according to their different sources, T and B cell superantigens can be divided into endogenous (viral) antigens and exogenous (viral) superantigens. bacterial type) superantigens.
Acute hepatitis has also been previously reported in children with multisystem inflammatory syndrome, but coinfection with other viruses has not been investigated. The researchers analyzed that the recent acute hepatitis of unknown cause in children may have been infected with the new coronavirus first, and the children were infected with adenovirus after the virus reservoir appeared in the intestine.
Specifically, as shown in the following figure: Schematic diagram of the pathology of adenovirus (AdV)-enhanced SARS-CoV-2 superantigen-mediated severe acute hepatitis.

Schematic illustration of the pathology of adenovirus (AdV)-enhanced SARS-CoV-2 superantigen-mediated severe acute hepatitis.
Following infection with 2019-nCoV, the viral reservoir may lead to repeated superantigen-mediated activation of immune cells over time, such as multisystem inflammatory syndrome (MIS-C). If such reservoirs exist and children are subsequently infected with adenovirus (AdV), this superantigen-mediated effect may be more pronounced and may lead to immunological abnormalities, as recently reported in acute severe hepatitis, which is why in these Cases should be investigated for evidence of this immunopathology.However, in the CDC sharing meeting, there was not much content about the investigation of serum new crown antibodies in children, and it was impossible to discuss the superantigen mechanism after new crown infection. Deepti Gurdasani, an epidemiologist at Queen Mary University of London, questioned, "I have to say that I was very surprised to see the recent CDC report on fulminant hepatitis in children. There has been so much discussion on the adenovirus issue, and the new crown serum has been mentioned by the way. Scientific investigation (but not yet completed). None of the liver biopsies found adenovirus, but the children were treated with antiviral drugs.”
 In a CDC sharing session, Elizabeth A. Moulton, an assistant professor at Texas Children's Hospital, mentioned the use of the antiviral drug cidofovir on some American children.
In a CDC sharing session, Elizabeth A. Moulton, an assistant professor at Texas Children's Hospital, mentioned the use of the antiviral drug cidofovir on some American children.Available data show that cidofovir is an open-loop nucleotide analog and is a new drug against cytomegalovirus (CMV). For other herpes viruses, such as herpes simplex virus type I and type II (HSV-I, HSV-II), varicella-herpes virus (VZV), Epstein-Barr virus, herpes virus type 6 (HHV-6), adenovirus and human Papillomatosis (HPV) is very active.
"From my point of view as a clinical virologist, there are many arguments against why adenoviruses cause acute hepatitis," says Eckerle, which has important implications for treatment. Why? This means that whether antiviral therapy or immunosuppressive therapy (such as cortisol) should be used can have very different effects on a patient's clinical outcome.
If the new coronavirus superantigen is considered, hormonal immunosuppressive therapy should be used. In fact, the latest May issue of JPGN REPORTS, a journal of paediatric gastroenterology and hepatology, reported a rare case of a healthy girl who contracted COVID-19 with mild symptoms but then developed acute liver failure. Doctors at Cincinnati Children's Hospital Medical Center and the University of Cincinnati School of Medicine use intravenous methylprednisolone to avoid liver transplants.
Even taking a step back and assuming adenovirus plays a role here, isn't it more likely that it's still an inflammatory response after infection, given the absence of adenovirus in the liver, Gurdasani said? Shouldn't treatment target this (with immunosuppression)?

"If the CDC doesn't want to provide us with serological data on SARS-CoV-2 antibodies in children under these circumstances, that's fine," Jalali said, "but please stop promoting 'adenovirus hepatitis' + cidofovir as a treatment False assumption. This is malfeasance.”
Related Posts
0 Comments
Write A Comments