
A small wound can be life-threatening. Hemophilia, known as "glass man," is an X-linked recessive inherited bleeding disorder divided into hemophilia A and hemophilia B. The former is caused by a mutation in the coagulation factor VIII gene (FVIII), while the latter is caused by a lack of coagulation factor IX (FIX).
Up to now, the main treatment for hemophilia is intravenous infusion of clotting factor replacement therapy, but there is still no cure. On May 19, local time, The Lancet Haematology, an international authoritative journal of hematology, published online the first liver-targeted adeno-associated virus (AAV) hemophilia B gene therapy (BBM- H901 injection) clinical study results. Preliminary findings suggest that liver-targeted BBM-H901 is safe 1 year after infusion, and that carrier-derived blood concentrations of factor IX activity (FIX:C) are high enough to prevent bleeding events and enable small-scale hemorrhagic events. The need for replacement therapy is minimized in patients with Friend B. The findings support further research, the team said.
Preliminary findings suggest that liver-targeted BBM-H901 is safe 1 year after infusion, and that carrier-derived blood concentrations of factor IX activity (FIX:C) are high enough to prevent bleeding events and enable small-scale hemorrhagic events. The need for replacement therapy is minimized in patients with Friend B. The findings support further research, the team said.
The clinical study is a collaboration between Hematology Hospital of Chinese Academy of Medical Sciences (Institute of Hematology, Chinese Academy of Medical Sciences), innovative pharmaceutical company Faith Medicine and East China University of Science and Technology. The corresponding authors are Prof. Zhang Lei and Prof. Yang Renchi from the Hematology Hospital of the Chinese Academy of Medical Sciences (Institute of Hematology, Chinese Academy of Medical Sciences) and Xiao Xiao, the co-founder, chairman and chief scientific officer of Faith Medicine.
The research team said that bleeding in patients with hemophilia B is closely related to the amount of coagulation factor IX activity (FIX:C) in the blood. Normal plasma coagulation factor IX activity is generally 50-120 IU/dl, while FIX activity in patients with severe hemophilia B is basically zero (<1IU/dl), which may occur in skin, joints, muscles, mucous membranes, internal organs, etc. Spontaneous bleeding. When the FIX:C level is > 20-40%, there will be no spontaneous bleeding.
BBM-H901, developed and produced by Faith Medicine, is a molecularly engineered adeno-associated virus (AAV) gene therapy drug candidate for hemophilia B. According to reports, Faith Medicine has independent intellectual property rights of AAV capsids and expression cassettes. The innovative human liver-targeted AAV capsid can efficiently infect hepatocytes, greatly shorten the residence time of drugs in the blood, and reduce the immune response generated by the capsid. The innovative expression cassette utilizes a mini liver-specific promoter to drive the high-efficiency expression of FIX-padua.
In this clinical study, a total of 10 severe/moderately severe hemophilia B patients (FIX:C<2%) were included, which is the first clinical trial of hemophilia B gene therapy using liver-targeted AAV as a vector in Asia Research. Before receiving treatment, the 10 patients had an average of 21.5 bleeding times (range: 3-60 times), 54 times of coagulation factor injections (range: 16-90 times) per year, and the activity of coagulation factor IX in the blood was below 5, and more The joints have developed lesions.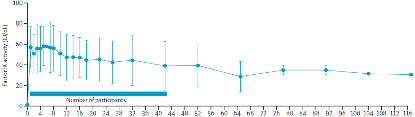
According to the research team, the gene therapy drug in this study has a rapid onset of action, and the curative effect can be obtained within 24 hours of infusion. The activity level of carrier-derived FIX in patients reached an average of 57.1 ± 20.2 IU/dl (mean ± SD) at one week, and FIX was reached at a median of 5 (1, 6) weeks: the highest value was 64.1 ± 22.5 IU/dl (mean ± SD).
After treatment, BBM-H901 brought significant clinical benefits to patients. The median annualized bleeding rate decreased from 12 to 0; the median number of target joints decreased from 1.5 to 0; and the median number of FIX infusions decreased from 53.5 to 0. In addition, BBM-H901 also demonstrated a favorable safety profile, with no grade 3-4 adverse events (AEs) occurring throughout the follow-up.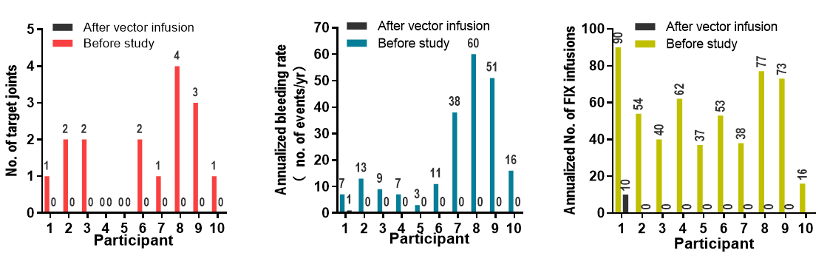
Professor Zhang Lei, deputy director of the Hematology Hospital of the Chinese Academy of Medical Sciences (Institute of Hematology, Chinese Academy of Medical Sciences), said, "The BBM-H901 study is the first clinical study in China and even Asia to use liver-targeted AAV vectors to treat hemophilia B. The safety, long-term effectiveness, and significant relief of related complications of the treatment strategy have all been confirmed, which will surely provide a reliable basis and theoretical support for the clinical application of systemic gene therapy drugs in the future, and will help promote the development and clinical transformation of gene therapy drugs in my country. It has important guiding significance.”
Up to now, the main treatment for hemophilia is intravenous infusion of clotting factor replacement therapy, but there is still no cure. On May 19, local time, The Lancet Haematology, an international authoritative journal of hematology, published online the first liver-targeted adeno-associated virus (AAV) hemophilia B gene therapy (BBM- H901 injection) clinical study results.
 Preliminary findings suggest that liver-targeted BBM-H901 is safe 1 year after infusion, and that carrier-derived blood concentrations of factor IX activity (FIX:C) are high enough to prevent bleeding events and enable small-scale hemorrhagic events. The need for replacement therapy is minimized in patients with Friend B. The findings support further research, the team said.
Preliminary findings suggest that liver-targeted BBM-H901 is safe 1 year after infusion, and that carrier-derived blood concentrations of factor IX activity (FIX:C) are high enough to prevent bleeding events and enable small-scale hemorrhagic events. The need for replacement therapy is minimized in patients with Friend B. The findings support further research, the team said.The clinical study is a collaboration between Hematology Hospital of Chinese Academy of Medical Sciences (Institute of Hematology, Chinese Academy of Medical Sciences), innovative pharmaceutical company Faith Medicine and East China University of Science and Technology. The corresponding authors are Prof. Zhang Lei and Prof. Yang Renchi from the Hematology Hospital of the Chinese Academy of Medical Sciences (Institute of Hematology, Chinese Academy of Medical Sciences) and Xiao Xiao, the co-founder, chairman and chief scientific officer of Faith Medicine.
The research team said that bleeding in patients with hemophilia B is closely related to the amount of coagulation factor IX activity (FIX:C) in the blood. Normal plasma coagulation factor IX activity is generally 50-120 IU/dl, while FIX activity in patients with severe hemophilia B is basically zero (<1IU/dl), which may occur in skin, joints, muscles, mucous membranes, internal organs, etc. Spontaneous bleeding. When the FIX:C level is > 20-40%, there will be no spontaneous bleeding.
BBM-H901, developed and produced by Faith Medicine, is a molecularly engineered adeno-associated virus (AAV) gene therapy drug candidate for hemophilia B. According to reports, Faith Medicine has independent intellectual property rights of AAV capsids and expression cassettes. The innovative human liver-targeted AAV capsid can efficiently infect hepatocytes, greatly shorten the residence time of drugs in the blood, and reduce the immune response generated by the capsid. The innovative expression cassette utilizes a mini liver-specific promoter to drive the high-efficiency expression of FIX-padua.
In this clinical study, a total of 10 severe/moderately severe hemophilia B patients (FIX:C<2%) were included, which is the first clinical trial of hemophilia B gene therapy using liver-targeted AAV as a vector in Asia Research. Before receiving treatment, the 10 patients had an average of 21.5 bleeding times (range: 3-60 times), 54 times of coagulation factor injections (range: 16-90 times) per year, and the activity of coagulation factor IX in the blood was below 5, and more The joints have developed lesions.

BBM-H901-derived FIX:C long-term expression levels.
After receiving gene therapy, i.e. a single intravenous infusion of 5 x 1012 vg/kg BBM-H901 injection, after a median follow-up of 58 weeks (range: 50-117 weeks), the vector-derived FIX activity level in patients reached an average of 36.9 ±20.5IU/dl (mean ±SD).According to the research team, the gene therapy drug in this study has a rapid onset of action, and the curative effect can be obtained within 24 hours of infusion. The activity level of carrier-derived FIX in patients reached an average of 57.1 ± 20.2 IU/dl (mean ± SD) at one week, and FIX was reached at a median of 5 (1, 6) weeks: the highest value was 64.1 ± 22.5 IU/dl (mean ± SD).
After treatment, BBM-H901 brought significant clinical benefits to patients. The median annualized bleeding rate decreased from 12 to 0; the median number of target joints decreased from 1.5 to 0; and the median number of FIX infusions decreased from 53.5 to 0. In addition, BBM-H901 also demonstrated a favorable safety profile, with no grade 3-4 adverse events (AEs) occurring throughout the follow-up.

BBM-H901 injection brought significant clinical benefits to patients.
The research team concluded that BBM-H901 injection has proven its safety and efficacy in the Chinese population, achieved long-term treatment of hemophilia B, alleviated the occurrence of related complications, and significantly increased patient benefits.Professor Zhang Lei, deputy director of the Hematology Hospital of the Chinese Academy of Medical Sciences (Institute of Hematology, Chinese Academy of Medical Sciences), said, "The BBM-H901 study is the first clinical study in China and even Asia to use liver-targeted AAV vectors to treat hemophilia B. The safety, long-term effectiveness, and significant relief of related complications of the treatment strategy have all been confirmed, which will surely provide a reliable basis and theoretical support for the clinical application of systemic gene therapy drugs in the future, and will help promote the development and clinical transformation of gene therapy drugs in my country. It has important guiding significance.”

On December 22, 2020, two brothers with hemophilia B received gene therapy, and each required an average of 45 injections of coagulation factor per year before treatment. After receiving gene therapy, the coagulation factors in the body have almost returned to normal, and no bleeding has occurred so far during the follow-up, and no further infusion of coagulation factors is required. At present, he has returned to normal work and life. Figure: Hematology Hospital, Chinese Academy of Medical Sciences (Institute of Hematology, Chinese Academy of Medical Sciences)
Dr. Zheng Jing, CEO of Faith Pharma, said, "This is China's first self-developed intravenous gene therapy drug for hemophilia B and a systemically delivered gene therapy drug for rare diseases. We will continue to advance all other drug candidates. pipeline discovery and research, and accelerate clinical trials and commercialization of drugs. We hope that patients with rare or common diseases will have access to gene therapy as soon as possible.”Related Posts
0 Comments
Write A Comments