Recently, a team from the University of Science and Technology of China constructed an oxygen-tolerant photoredox-catalyzed strategy for regulating NO release, which can be used in the treatment of bacterial infections.
Nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) are endogenous gaseous transmitters with important physiological or pathological functions, as well as excellent anti-inflammatory and antibacterial activities. In view of the unique advantages and clear efficacy of gas transmitters in the treatment of cardiovascular and cerebrovascular diseases and respiratory diseases (such as new coronary pneumonia), the development of gas transmitter drugs has received extensive attention.
Unlike traditional solid and liquid drugs, gaseous mediators cannot be administered orally, injected, or transdermally. In addition, gas transmitters are easily metabolized under physiological conditions and have a very short half-life. After inhalation administration, they mainly act on the lungs and are difficult to reach other lesions.
In response to the aforementioned problems, Hu Jinming's research group from the School of Chemistry and Materials Science, University of Science and Technology of China has carried out a series of work on the controllable delivery of gas transmitters, and initially explored the potential applications of gas transmitter polymer materials in the treatment of inflammatory and infectious diseases .
This time, Hu Jinming's research group cooperated with Professor Liu Shiyong's research group and Xiao Shiyan's research group to study an oxygen-tolerant photoredox catalysis strategy for regulating NO release, which was successfully used in the treatment of bacterial infections.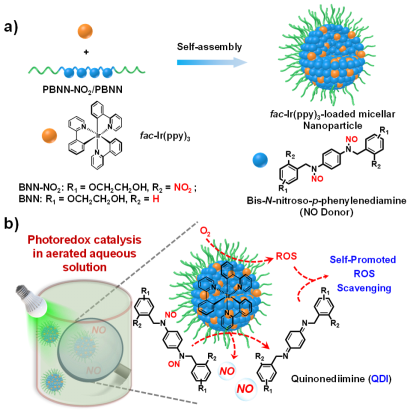
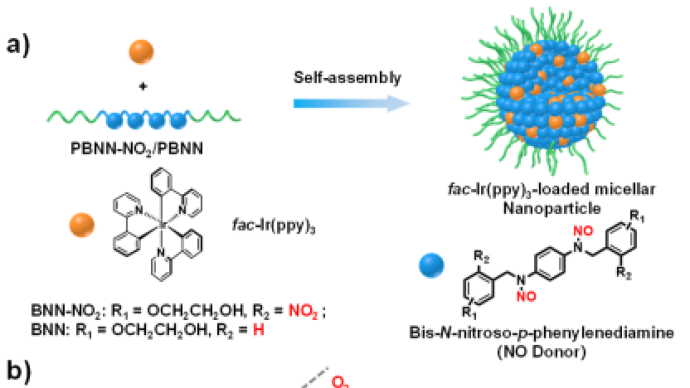
The team found that BNN-NO2 and BNN can be spontaneously converted into the corresponding quinodiimide (QDI) structure after NO release, and the generated reactive oxygen species (ROS) can be scavenged in situ, achieving a unique oxygen tolerance performance. Although photoredox catalysis has been widely used in organic synthesis, the utilization of photoredox catalysis to activate bioactive molecules in physiological environments still faces great challenges. This is due to the complexity of the physiological environment, and photoredox catalytic reactions are often affected by surrounding biomolecules. For example, oxygen can severely quench the excited states of photocatalysts.
The researchers introduced BNN-NO2 or BNN into the amphiphilic block polymer through copolymerization, and after loading the photocatalyst fac-Ir(ppy)3, uniform micellar nanoparticles could be obtained. Under visible light irradiation, the triggered release of NO was successfully achieved without the addition of additional oxygen scavengers, accompanied by a spontaneous decrease in the dissolved oxygen content in water.
The results of in vitro antibacterial experiments show that the aforementioned NO-releasing micelles can hyperpolarize the bacterial membrane potential and increase the permeability of the bacterial membrane to achieve the effect of killing bacteria. In a mouse full-thickness bacterial infection wound model, photoredox catalysis triggers NO release to effectively treat methicillin-resistant Staphylococcus aureus (MRSA) wound infection and promote wound healing.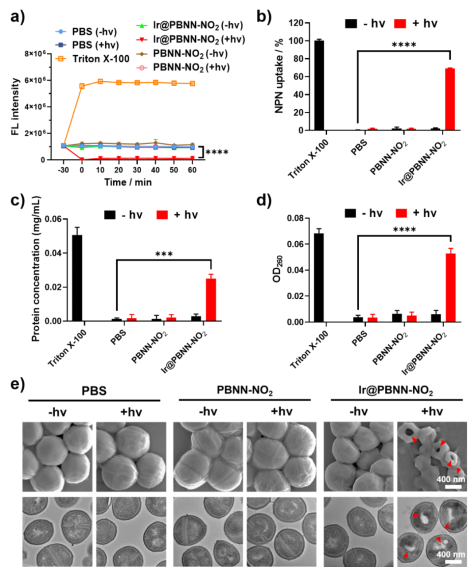
Nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) are endogenous gaseous transmitters with important physiological or pathological functions, as well as excellent anti-inflammatory and antibacterial activities. In view of the unique advantages and clear efficacy of gas transmitters in the treatment of cardiovascular and cerebrovascular diseases and respiratory diseases (such as new coronary pneumonia), the development of gas transmitter drugs has received extensive attention.
Unlike traditional solid and liquid drugs, gaseous mediators cannot be administered orally, injected, or transdermally. In addition, gas transmitters are easily metabolized under physiological conditions and have a very short half-life. After inhalation administration, they mainly act on the lungs and are difficult to reach other lesions.
In response to the aforementioned problems, Hu Jinming's research group from the School of Chemistry and Materials Science, University of Science and Technology of China has carried out a series of work on the controllable delivery of gas transmitters, and initially explored the potential applications of gas transmitter polymer materials in the treatment of inflammatory and infectious diseases .
This time, Hu Jinming's research group cooperated with Professor Liu Shiyong's research group and Xiao Shiyan's research group to study an oxygen-tolerant photoredox catalysis strategy for regulating NO release, which was successfully used in the treatment of bacterial infections.

Figure 1: Construction of an oxygen-tolerant photoredox catalysis-driven nitric oxide delivery system, the picture comes from the University of Science and Technology of China
The team used UV light-absorbing N,N′-dinitroso-1,4-phenylenediamine light-responsive NO-releasing molecules (BNN-NO2 and BNN) as the research object (as shown in Figure 1). Studies have shown that BNN-NO2 and BNN are activated by the photocatalyst tris(2-phenylpyridine)iridium (fac-Ir(ppy)3) under visible light (500 nm) irradiation and release NO.The team found that BNN-NO2 and BNN can be spontaneously converted into the corresponding quinodiimide (QDI) structure after NO release, and the generated reactive oxygen species (ROS) can be scavenged in situ, achieving a unique oxygen tolerance performance. Although photoredox catalysis has been widely used in organic synthesis, the utilization of photoredox catalysis to activate bioactive molecules in physiological environments still faces great challenges. This is due to the complexity of the physiological environment, and photoredox catalytic reactions are often affected by surrounding biomolecules. For example, oxygen can severely quench the excited states of photocatalysts.
The researchers introduced BNN-NO2 or BNN into the amphiphilic block polymer through copolymerization, and after loading the photocatalyst fac-Ir(ppy)3, uniform micellar nanoparticles could be obtained. Under visible light irradiation, the triggered release of NO was successfully achieved without the addition of additional oxygen scavengers, accompanied by a spontaneous decrease in the dissolved oxygen content in water.
The results of in vitro antibacterial experiments show that the aforementioned NO-releasing micelles can hyperpolarize the bacterial membrane potential and increase the permeability of the bacterial membrane to achieve the effect of killing bacteria. In a mouse full-thickness bacterial infection wound model, photoredox catalysis triggers NO release to effectively treat methicillin-resistant Staphylococcus aureus (MRSA) wound infection and promote wound healing.

Figure 2: Photoredox catalysis triggers the release of nitric oxide to achieve cell membrane hyperpolarization and enhanced bacterial membrane permeability, effectively killing methicillin-resistant Staphylococcus aureus (MRSA). Picture from University of Science and Technology of China
The aforementioned work provides a useful reference for exploring the biomedical applications of photoredox catalysis, and its unique oxygen tolerance effectively avoids the adverse effects of oxygen on photoredox catalysis in physiological environments. The research was funded by the National Natural Science Foundation of China, the Key R&D Program of the Ministry of Science and Technology, and the Cultivation Fund of the University of Science and Technology of China.Related Posts
0 Comments
Write A Comments