Since May, a rare and unusual monkeypox outbreak has broken out in many countries around the world. WHO notified on May 24 that 131 confirmed and 106 suspected cases of monkeypox have been reported in 19 countries. The outbreak is unusual but controllable. In 1980, WHO declared that smallpox had been eradicated globally, and monkeypox is considered the most important orthopoxvirus infection in humans since the eradication of smallpox.
Before the current outbreak, monkeypox cases were mainly scattered in the tropical rainforests of central and western Africa. What are the characteristics of monkeypox infected patients? What treatment experience do you have? An infectious disease research team from the University of Liverpool, UK, studied the clinical characteristics and treatment course of seven monkeypox patients diagnosed in the UK between 2018 and 2021. Research suggests that some antiviral drugs used to treat smallpox or animal-borne monkeypox may slow monkeypox symptoms in humans and shorten the length of time a patient is infected.
At 6:30 on May 25, Beijing time, the international authoritative academic journal "The Lancet Infectious Diseases" published the research results in the form of a paper (Clinical characteristics and disease management of monkeypox cases in the UK: a retrospective observational study). , "Clinical features and management of human monkeypox: a retrospective observational study in the UK"). The latest viral genome sequencing shows that the monkeypox virus that caused this outbreak is homologous to the monkeypox virus discovered in the UK in 2018. As optimal infection control and treatment strategies for monkeypox have not yet been established, data from this study can inform further global understanding of the disease's clinical features and transmission dynamics.
The latest viral genome sequencing shows that the monkeypox virus that caused this outbreak is homologous to the monkeypox virus discovered in the UK in 2018. As optimal infection control and treatment strategies for monkeypox have not yet been established, data from this study can inform further global understanding of the disease's clinical features and transmission dynamics.
The aforementioned study is the first to analyse nosocomial and household transmission of human monkeypox in the UK between 2018 and 2021, while reporting patient exposure to two different antiviral drugs, brincidofovir and brincidofovir. Response to tecovirimat treatment. Both drugs have previously been primarily used to treat smallpox and have demonstrated some efficacy against monkeypox in animals.
The study found that tecovirimat may reduce the duration of monkeypox symptoms in humans and shorten the time of contagion in patients, the potential of which needs further study, while there is little evidence that brincidofovir has clinical curative effect. The paper also reported finding monkeypox virus in blood and throat swabs from patients. All patients in the study recovered after being treated in isolation in UK hospitals.
Additionally, during previous monkeypox outbreaks, patients were considered contagious until all lesions had crusted over. In their study of the seven UK cases, the researchers found that patients observed viral shedding for at least three weeks after infection.
"Currently, global public health authorities are trying to understand the causes of monkeypox outbreaks in Europe and North America since May of this year - with many cases having neither travel history nor clear links to known cases, our study provides some insight into the use of "Preliminary insights into the treatment of monkeypox with antiviral drugs," said lead author Dr Hugh Adler of Liverpool University Hospitals NHS Foundation Trust, "although the recent outbreak has seen more cases than we have previously encountered in the UK. More, but historically the monkeypox virus has not spread very efficiently from person to person, and overall the risk to public safety is low."
Monkeypox, a close relative of the smallpox virus, is a rare disease, but it is classified as a High Consequence Infectious Disease (HCID) by the UK Health Security Agency. Currently, there is no licensed monkeypox treatment globally, and data on the duration of its infectivity is extremely limited, with the virus generally believed to have an incubation period ranging from 5 to 21 days. Patients are usually isolated in specialist hospitals to prevent the spread of the virus.
Monkeypox is spread from animals to people, usually as a result of animal bites or eating improperly cooked meat. In rare cases, the virus can spread from person to person. The first human cases of monkeypox were reported in the Democratic Republic of the Congo in 1970, and rarely occur outside the countries of Central and West Africa. To date, there has also been little research on monkeypox cases in high-income countries.
Currently, monkeypox symptoms reported globally include fever, rash, and swollen lymph nodes. Complications have also been reported, including lung inflammation, brain inflammation, vision-threatening corneal inflammation, and secondary bacterial infections. Published mortality rates vary widely, ranging from 1-10% in Congo Basin cases and below 3% in Nigerian cases. Most of the deaths from monkeypox are children and people living with HIV. Two oral drugs, brincidofovir and tecovirimat, have been approved for the treatment of smallpox and have been shown to be effective against monkeypox in animals.
In this retrospective observational study, investigators compared confirmed monkeypox (defined as monkeypox (defined as a All patients (7) with poxvirus PCR-positive clinical disease) underwent a chart review. The investigators extracted clinical data (including demographic variables, development of symptoms and signs, disease complications, and any antiviral treatment received) and laboratory results (including routine biochemical testing and monkeypox virus PCR) for comparison.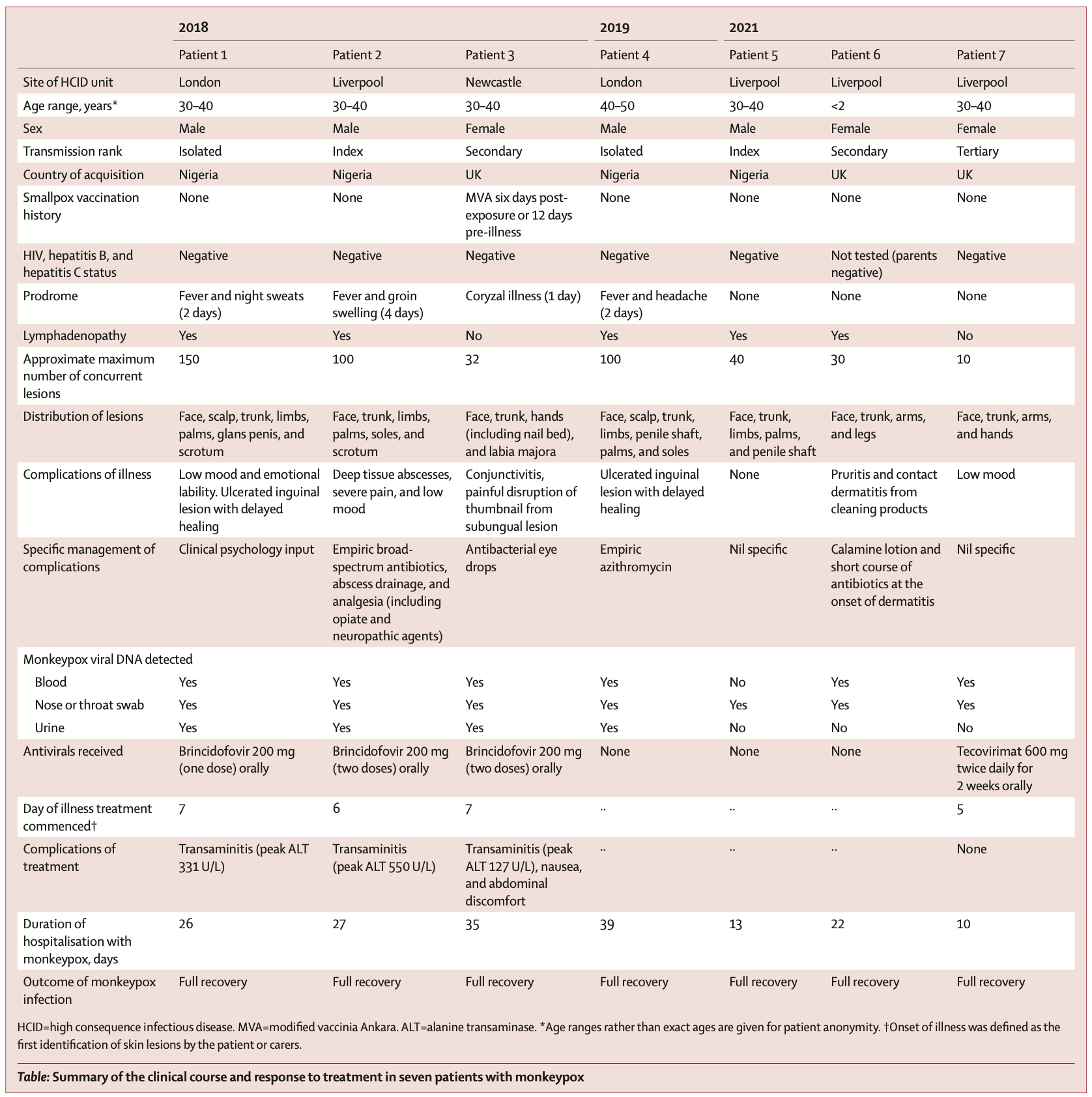 Among them, PCR detection of monkeypox virus in patients was completed in the UK Rare and Imported Pathogens Laboratory (UK Rare and Imported Pathogens Laboratory). Test samples include EDTA (ethylenediaminetetraacetic acid) blood samples, urine samples, fluid swabs from persistent lesions or lesions, and upper respiratory tract swabs. Testing is usually performed every 48-72 hours until each anatomical site (i.e. skin , blood or respiratory tract) record two consecutive negative results. These negative results, combined with peeling of all visible lesions, no new lesions, and no active mucosal lesions, constitute the standard framework for HCID centers to agree to send patients to community isolation.
Among them, PCR detection of monkeypox virus in patients was completed in the UK Rare and Imported Pathogens Laboratory (UK Rare and Imported Pathogens Laboratory). Test samples include EDTA (ethylenediaminetetraacetic acid) blood samples, urine samples, fluid swabs from persistent lesions or lesions, and upper respiratory tract swabs. Testing is usually performed every 48-72 hours until each anatomical site (i.e. skin , blood or respiratory tract) record two consecutive negative results. These negative results, combined with peeling of all visible lesions, no new lesions, and no active mucosal lesions, constitute the standard framework for HCID centers to agree to send patients to community isolation.
In addition, the Bundeswehr Institute for Microbiology (Munich, Germany) performed orthopoxvirus IgG and IgM detection by immunofluorescence in the sera of 4 people who had been exposed to monkeypox.
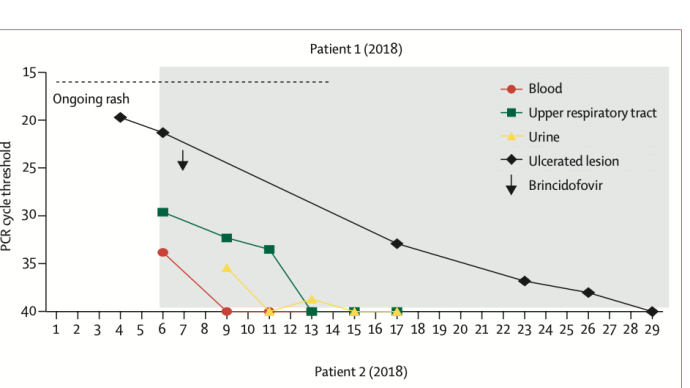
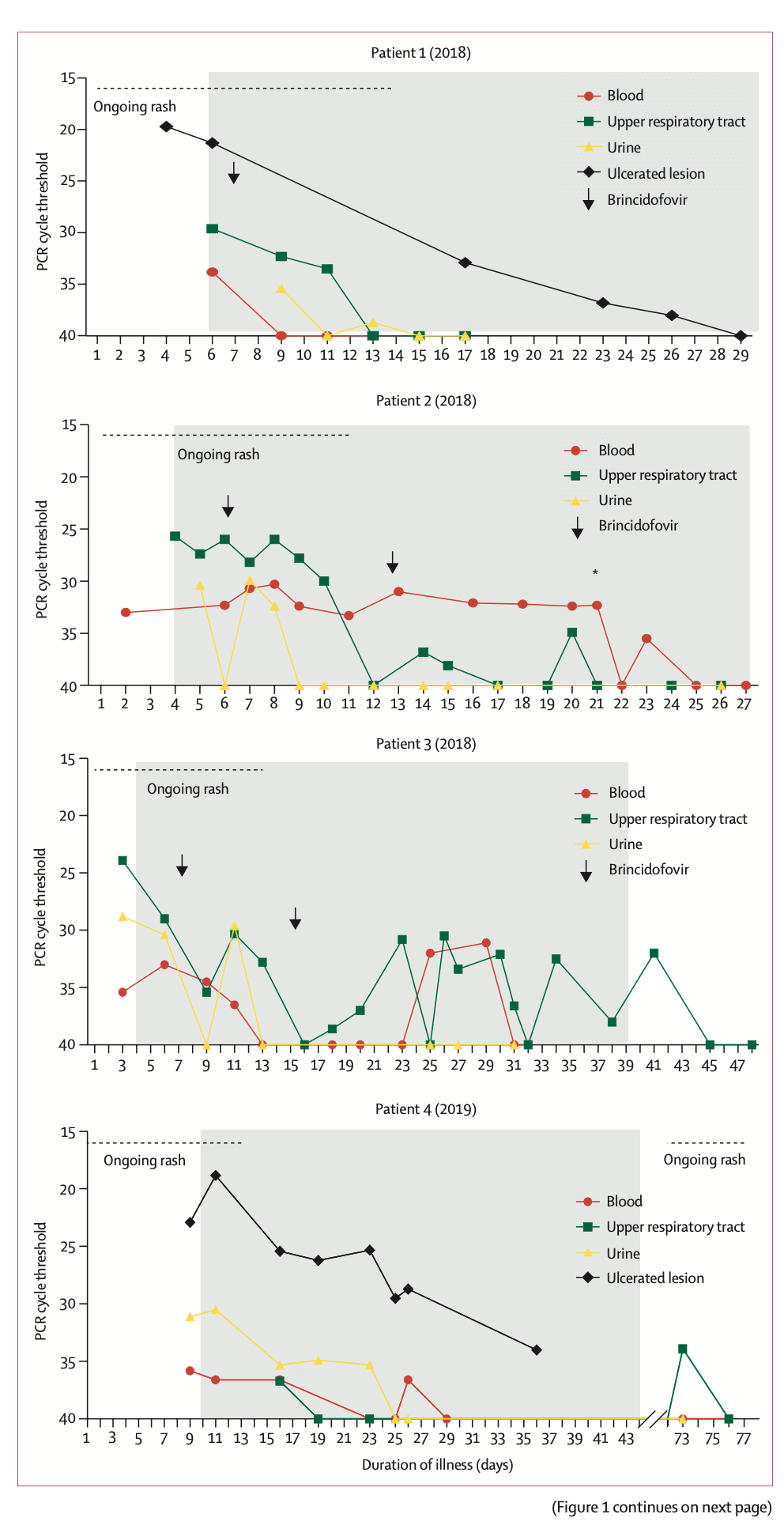
The researchers reported the clinical symptoms and treatment course of the seven patients with graphs. Of these 7 patients, 4 were infected with monkeypox outside the UK (patients 1, 2, 4 and 5); 3 were infected within the UK (patients 3, 6, 7). In terms of time, 4 cases occurred in 2018-2019 and 3 cases occurred in 2021. All seven patients exhibited pleomorphic skin lesions (including papules, vesicles, pustules, umbilical pus, ulcerative lesions, and crusts), and the lesions were positive for monkeypox virus PCR. Viral DNA was detected in the upper respiratory tract swabs of all patients, and in the blood of 6 patients and the urine of 4 patients.
All seven patients exhibited pleomorphic skin lesions (including papules, vesicles, pustules, umbilical pus, ulcerative lesions, and crusts), and the lesions were positive for monkeypox virus PCR. Viral DNA was detected in the upper respiratory tract swabs of all patients, and in the blood of 6 patients and the urine of 4 patients. 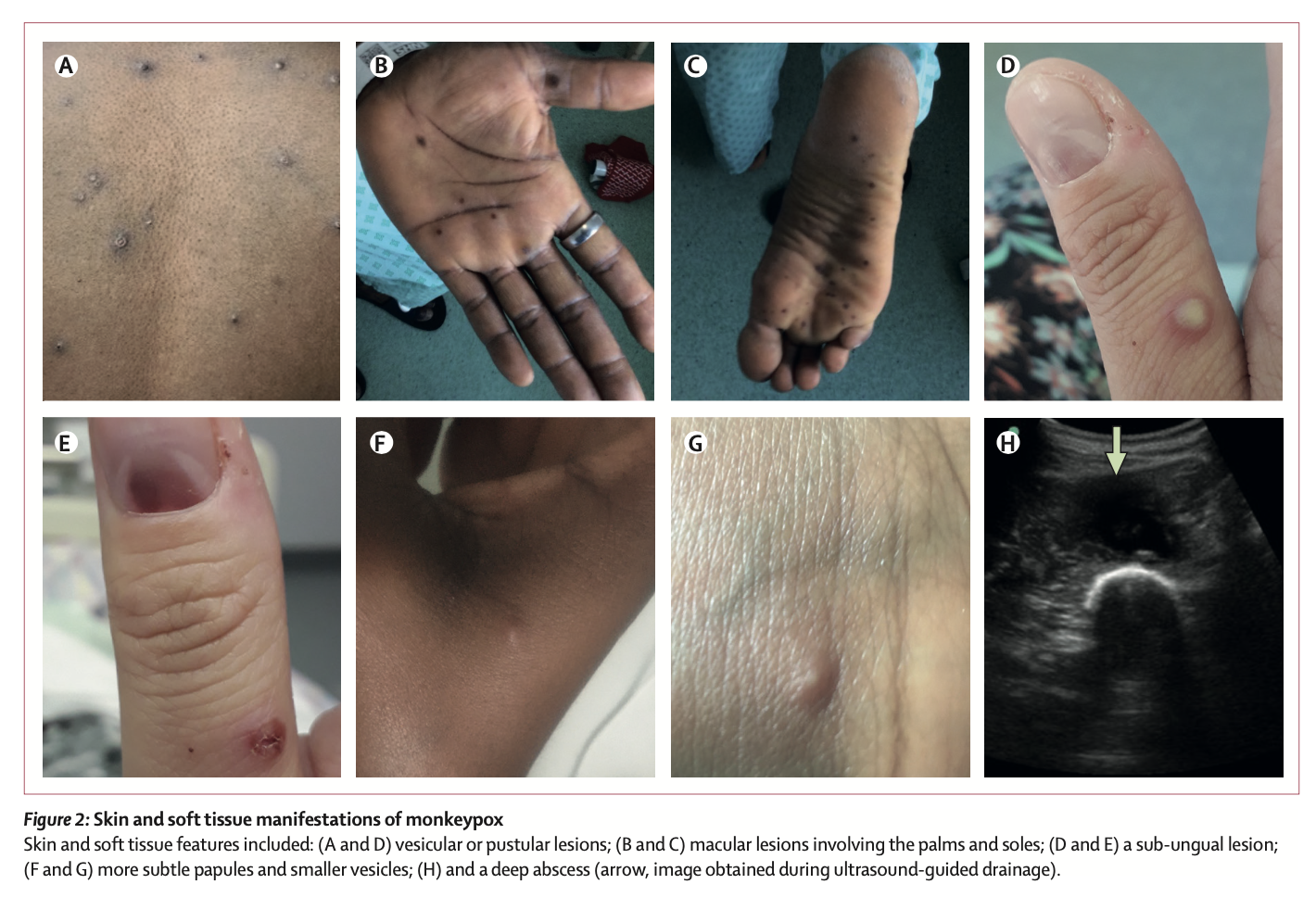 Between 2018 and 2019, the four patients the study looked at were treated for monkeypox at HCID centres in the UK. Three of the cases were imported from West Africa, and the fourth was a health care worker 18 days after initial exposure to the virus, the first case of monkeypox transmission in a hospital setting outside Africa.
Between 2018 and 2019, the four patients the study looked at were treated for monkeypox at HCID centres in the UK. Three of the cases were imported from West Africa, and the fourth was a health care worker 18 days after initial exposure to the virus, the first case of monkeypox transmission in a hospital setting outside Africa.
The first three patients were treated with brincidofovir 7 days after the onset of the rash. The proposed treatment course was 200 mg three times a week, but all 3 patients had elevated alanine aminotransferase after taking the drug, and none of them completed the full course of treatment. The scientists did not observe any convincing clinical benefit of brincidofovir in the treatment of monkeypox. Nonetheless, all three patients, as well as the fourth patient with nosocomial transmission, fully recovered after 45-48 days.
Patient 4 (2019) developed a recurrence after discharge. He had sexual intercourse about 6 weeks after being discharged from the hospital, after which the lymph nodes enlarged. However, the recurrence time of the patient's symptoms was short, and the clinical performance was good in other aspects. He was temporarily admitted to the local hospital until the lesions crusted and the upper respiratory tract swab PCR test was negative and then discharged.
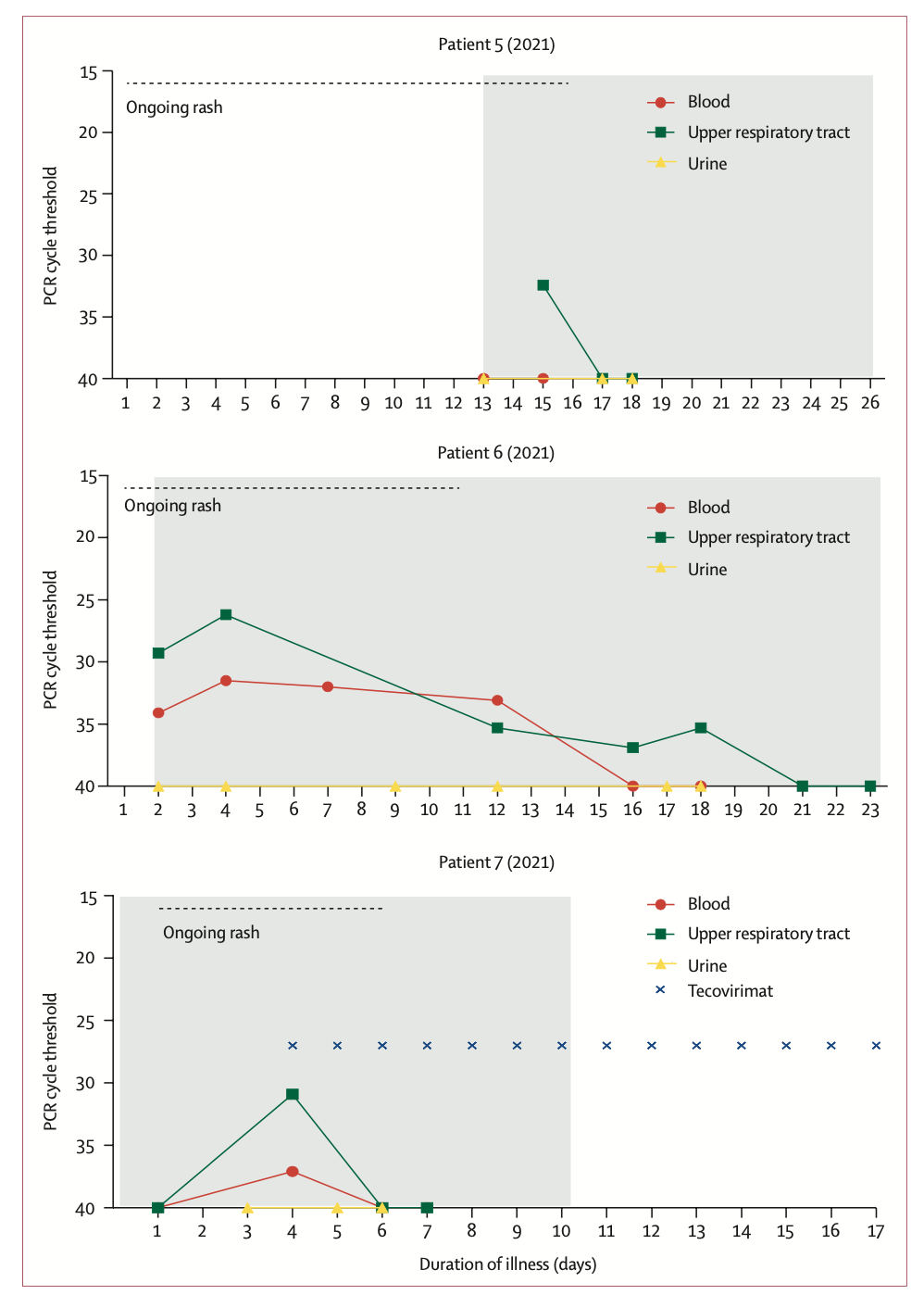
Of particular note are the family monkeypox clusters that occurred in 2021. The family (father, mother and four children under 10) came to the UK from Nigeria. During a 10-day period of mandatory self-isolation with COVID-19, the father (patient 5 [2021]) developed a progressive vesicular rash, which he believed was caused by chickenpox. After the quarantine period ended, he visited the local emergency room and was admitted to the regional HCID center, where he was diagnosed with monkeypox by vesicular fluid PCR testing.
When the youngest child subsequently developed fever and blisters, the whole family was admitted to the same HCID ward. The three older siblings were grouped with their father, who was thought to be non-infectious at the time, and the three children and their father were discharged after blood and upper respiratory tract swabs were taken and PCR was negative for monkeypox virus. Completed 21-day quarantine at home.
The mother of the family requested to be hospitalized and continued to care for her daughter (Patient 6 [2021]). After the child's monkeypox was confirmed by PCR testing of lesion swabs, medical personnel considered taking tecovirimat for treatment, but considering that the drug was not approved for use in children at the time, and it was not suitable for children weighing less than 13 kg. The patient did not have a standardized dose and had only previously been given it in a child with smallpox infection, so it was not used. After the 20th day of onset, the PCR test for monkeypox virus in the upper respiratory tract swab of patient 6 was negative.
However, on day 14 of the onset of patient 6 (2021), the mother (patient 7 [2021]) developed headache, pharyngitis, and chest vesicles, and the monkeypox DNA PCR test was positive. In the mother's isolation, the team decided to offer a 2-week course of oral tecovirimat (600 mg twice daily). The aim of treatment is to prevent complications and shorten the length of hospital stay. Samples from the patient's blood and upper respiratory tract were PCR negative 48 hours after taking tecovirimat and remained negative at 72 hours. No new lesions appeared 24 hours after tecovirimat treatment.
The choice of the two drugs during treatment is undoubtedly the most concerning. In this regard, the researchers commented, "When the first monkeypox patients were diagnosed in the UK in 2018, brincidofovir and tecovirimat were not licensed for the treatment of monkeypox. Brincidofovir was selected in 2018 because of the reuse of supplies already in local clinical trials by medical personnel in an emergency situation. Given the small number of people involved, it is difficult to infer bricindofovir The relationship between fovir treatment and disease course. Although in several samples, monkeypox virus PCR cycle thresholds were transiently decreased after patients took the drug, these improvements were not durable and were also seen between patients. Not consistent. We do not know whether administration earlier in the disease course or with a different dosing regimen would have resulted in more beneficial clinical outcomes with brincidofovir. All 3 patients experienced post-treatment Disturbance of liver enzymes, which directly led to the decision to reduce the duration of medication. However, in the treatment of prairie dogs with confirmed monkeypox infection, brincidofovir had a modest survival benefit (29% vs 14%), and reduced viral titers in end organs."
"The use of tecovirimat in the treatment of patient 7 (2021) is based on the evidence of efficacy of the drug in animal models of orthopoxvirus infection and the drug's good tolerance in humans. Physicians hope This treatment regimen prevents disease progression or shortens the length of hospital stay. Treatment begins shortly after skin lesions develop. Tecoviride compared to patients not taking the drug or those treated with brincidofovir The clinical and virological responses of (tecovirimat) were faster and time-correlated, however, we were not sure whether this was the result of tecovirimat treatment. In the treatment of smallpox-infected macaques, compared with placebo, Tecovirimat was also effective in reducing the number of lesions in the blood and upper respiratory tract and shortening the duration of PCR positivity. Available data suggest that a 5-day course of tecovirimat is sufficient to produce clinical responses, whereas A 2-week course of treatment produces humoral immunity and durable virus clearance.
In addition, the researchers emphasized in the paper that great attention should be paid to children infected with monkeypox. Based on historical data, monkeypox infection in children is more likely to be severely ill than adults, and is also more associated with death, they said. Patient 6 (2021) is the first pediatric patient with monkeypox reported outside Africa since 2003 and the first pediatric patient managed by the UK HCID network. During treatment, a paediatric team from the UK Department of Child Health and Social Development provided on-site care in an adult isolation ward, addressing the challenges of managing a young child and an adult in isolation. The pediatric patient required careful multidisciplinary and interprofessional coordination, collaboration, and communication, and fortunately, this pediatric patient had a milder disease course.
On the viral dynamics of human monkeypox infection, the researchers stated, "In previous monkeypox cases and outbreaks, patients were thought to be infectious until all lesions had crusted over. In these seven patients' disease course follow-up , we observed trajectories of positive PCR in patient blood and upper respiratory tract swabs, similar to non-human primate models of monkeypox and smallpox infection. It took at least 3 weeks for viral DNA shedding in upper respiratory swabs from the first 3 patients Time. The infectivity of patients at the time of upper respiratory tract swab positive and skin crusting lesions remains undetermined and is an important area for future research with immediate practical implications for health care resource use, safe patient discharge, and prevention of transmission.
In addition, for the recurrence of patient 4 (2019), the researchers believe that this is related to mild, short-term clinical disease and transient shedding of monkeypox virus DNA. The temporal correlation between sexual intercourse, increased inguinal lymph nodes, and rash recurrence may suggest that the genitals may act as a reservoir for monkeypox virus. But the theory warrants dedicated study in larger patient samples.
The paper also mentions, "All patients were young, had no pre-existing complications, and none had been vaccinated against smallpox prior to exposure to the virus. Nonetheless, most patients had a relatively mild course of disease, which is consistent with the monkeypox virus in West Africa. The infection is consistent with bronchial infection. Depressed mood is more common.”
None of the patients experienced any serious complications common to monkeypox, such as pneumonia or superimposed bacterial sepsis. Patient 2 (2018) represents the first adult patient report of a deep tissue monkeypox abscess. Neither of the patient's two abscesses directly communicated with the superficial skin lesions, and the thigh abscess was found by ultrasonography about 2 weeks after diagnosis, and the PCR cycle threshold for monkeypox virus in the abscess fluid was low.
Finally, the investigators acknowledge some limitations of the study, namely the observational nature and the small number of cases analyzed. The researchers were also unable to confirm a positive monkeypox PCR test with laboratory cultures of the virus, meaning that the circulating infectious virus could not be confirmed.
"The cases reported in our study, as well as the recent outbreak, highlight the importance of maintaining a global collaborative network that we need to manage sporadic outbreaks of high-consequence pathogens such as monkeypox." Liverpool, UK Dr Nick Price, University NHS Trust and one of the authors of the paper, said: "The cases we observed were extremely challenging to manage and difficult to manage even in a high-income setting like the UK. As international travel returns to pre-pandemic levels, public health officials and health care workers around the world must remain vigilant about the potential for new cases of monkeypox."
Attached: Chart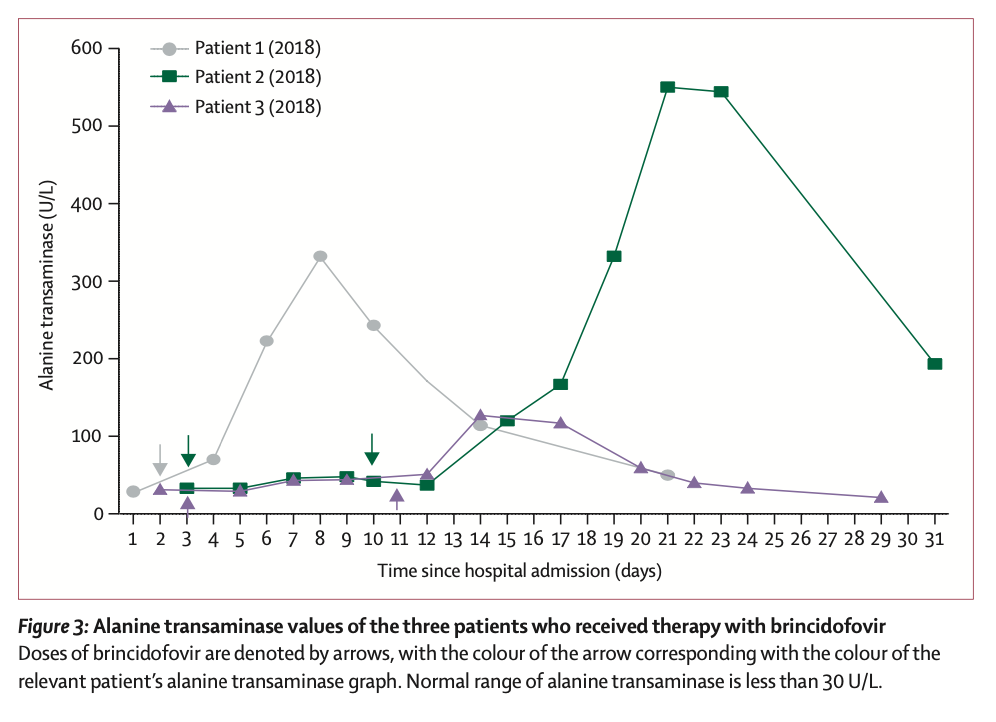
Before the current outbreak, monkeypox cases were mainly scattered in the tropical rainforests of central and western Africa. What are the characteristics of monkeypox infected patients? What treatment experience do you have? An infectious disease research team from the University of Liverpool, UK, studied the clinical characteristics and treatment course of seven monkeypox patients diagnosed in the UK between 2018 and 2021. Research suggests that some antiviral drugs used to treat smallpox or animal-borne monkeypox may slow monkeypox symptoms in humans and shorten the length of time a patient is infected.
At 6:30 on May 25, Beijing time, the international authoritative academic journal "The Lancet Infectious Diseases" published the research results in the form of a paper (Clinical characteristics and disease management of monkeypox cases in the UK: a retrospective observational study). , "Clinical features and management of human monkeypox: a retrospective observational study in the UK").
 The latest viral genome sequencing shows that the monkeypox virus that caused this outbreak is homologous to the monkeypox virus discovered in the UK in 2018. As optimal infection control and treatment strategies for monkeypox have not yet been established, data from this study can inform further global understanding of the disease's clinical features and transmission dynamics.
The latest viral genome sequencing shows that the monkeypox virus that caused this outbreak is homologous to the monkeypox virus discovered in the UK in 2018. As optimal infection control and treatment strategies for monkeypox have not yet been established, data from this study can inform further global understanding of the disease's clinical features and transmission dynamics.The aforementioned study is the first to analyse nosocomial and household transmission of human monkeypox in the UK between 2018 and 2021, while reporting patient exposure to two different antiviral drugs, brincidofovir and brincidofovir. Response to tecovirimat treatment. Both drugs have previously been primarily used to treat smallpox and have demonstrated some efficacy against monkeypox in animals.
The study found that tecovirimat may reduce the duration of monkeypox symptoms in humans and shorten the time of contagion in patients, the potential of which needs further study, while there is little evidence that brincidofovir has clinical curative effect. The paper also reported finding monkeypox virus in blood and throat swabs from patients. All patients in the study recovered after being treated in isolation in UK hospitals.
Additionally, during previous monkeypox outbreaks, patients were considered contagious until all lesions had crusted over. In their study of the seven UK cases, the researchers found that patients observed viral shedding for at least three weeks after infection.
"Currently, global public health authorities are trying to understand the causes of monkeypox outbreaks in Europe and North America since May of this year - with many cases having neither travel history nor clear links to known cases, our study provides some insight into the use of "Preliminary insights into the treatment of monkeypox with antiviral drugs," said lead author Dr Hugh Adler of Liverpool University Hospitals NHS Foundation Trust, "although the recent outbreak has seen more cases than we have previously encountered in the UK. More, but historically the monkeypox virus has not spread very efficiently from person to person, and overall the risk to public safety is low."
Monkeypox, a close relative of the smallpox virus, is a rare disease, but it is classified as a High Consequence Infectious Disease (HCID) by the UK Health Security Agency. Currently, there is no licensed monkeypox treatment globally, and data on the duration of its infectivity is extremely limited, with the virus generally believed to have an incubation period ranging from 5 to 21 days. Patients are usually isolated in specialist hospitals to prevent the spread of the virus.
Monkeypox is spread from animals to people, usually as a result of animal bites or eating improperly cooked meat. In rare cases, the virus can spread from person to person. The first human cases of monkeypox were reported in the Democratic Republic of the Congo in 1970, and rarely occur outside the countries of Central and West Africa. To date, there has also been little research on monkeypox cases in high-income countries.
Currently, monkeypox symptoms reported globally include fever, rash, and swollen lymph nodes. Complications have also been reported, including lung inflammation, brain inflammation, vision-threatening corneal inflammation, and secondary bacterial infections. Published mortality rates vary widely, ranging from 1-10% in Congo Basin cases and below 3% in Nigerian cases. Most of the deaths from monkeypox are children and people living with HIV. Two oral drugs, brincidofovir and tecovirimat, have been approved for the treatment of smallpox and have been shown to be effective against monkeypox in animals.
In this retrospective observational study, investigators compared confirmed monkeypox (defined as monkeypox (defined as a All patients (7) with poxvirus PCR-positive clinical disease) underwent a chart review. The investigators extracted clinical data (including demographic variables, development of symptoms and signs, disease complications, and any antiviral treatment received) and laboratory results (including routine biochemical testing and monkeypox virus PCR) for comparison.
 Among them, PCR detection of monkeypox virus in patients was completed in the UK Rare and Imported Pathogens Laboratory (UK Rare and Imported Pathogens Laboratory). Test samples include EDTA (ethylenediaminetetraacetic acid) blood samples, urine samples, fluid swabs from persistent lesions or lesions, and upper respiratory tract swabs. Testing is usually performed every 48-72 hours until each anatomical site (i.e. skin , blood or respiratory tract) record two consecutive negative results. These negative results, combined with peeling of all visible lesions, no new lesions, and no active mucosal lesions, constitute the standard framework for HCID centers to agree to send patients to community isolation.
Among them, PCR detection of monkeypox virus in patients was completed in the UK Rare and Imported Pathogens Laboratory (UK Rare and Imported Pathogens Laboratory). Test samples include EDTA (ethylenediaminetetraacetic acid) blood samples, urine samples, fluid swabs from persistent lesions or lesions, and upper respiratory tract swabs. Testing is usually performed every 48-72 hours until each anatomical site (i.e. skin , blood or respiratory tract) record two consecutive negative results. These negative results, combined with peeling of all visible lesions, no new lesions, and no active mucosal lesions, constitute the standard framework for HCID centers to agree to send patients to community isolation.In addition, the Bundeswehr Institute for Microbiology (Munich, Germany) performed orthopoxvirus IgG and IgM detection by immunofluorescence in the sera of 4 people who had been exposed to monkeypox.
The researchers reported the clinical symptoms and treatment course of the seven patients with graphs. Of these 7 patients, 4 were infected with monkeypox outside the UK (patients 1, 2, 4 and 5); 3 were infected within the UK (patients 3, 6, 7). In terms of time, 4 cases occurred in 2018-2019 and 3 cases occurred in 2021.
 All seven patients exhibited pleomorphic skin lesions (including papules, vesicles, pustules, umbilical pus, ulcerative lesions, and crusts), and the lesions were positive for monkeypox virus PCR. Viral DNA was detected in the upper respiratory tract swabs of all patients, and in the blood of 6 patients and the urine of 4 patients.
All seven patients exhibited pleomorphic skin lesions (including papules, vesicles, pustules, umbilical pus, ulcerative lesions, and crusts), and the lesions were positive for monkeypox virus PCR. Viral DNA was detected in the upper respiratory tract swabs of all patients, and in the blood of 6 patients and the urine of 4 patients.  Between 2018 and 2019, the four patients the study looked at were treated for monkeypox at HCID centres in the UK. Three of the cases were imported from West Africa, and the fourth was a health care worker 18 days after initial exposure to the virus, the first case of monkeypox transmission in a hospital setting outside Africa.
Between 2018 and 2019, the four patients the study looked at were treated for monkeypox at HCID centres in the UK. Three of the cases were imported from West Africa, and the fourth was a health care worker 18 days after initial exposure to the virus, the first case of monkeypox transmission in a hospital setting outside Africa.The first three patients were treated with brincidofovir 7 days after the onset of the rash. The proposed treatment course was 200 mg three times a week, but all 3 patients had elevated alanine aminotransferase after taking the drug, and none of them completed the full course of treatment. The scientists did not observe any convincing clinical benefit of brincidofovir in the treatment of monkeypox. Nonetheless, all three patients, as well as the fourth patient with nosocomial transmission, fully recovered after 45-48 days.
Patient 4 (2019) developed a recurrence after discharge. He had sexual intercourse about 6 weeks after being discharged from the hospital, after which the lymph nodes enlarged. However, the recurrence time of the patient's symptoms was short, and the clinical performance was good in other aspects. He was temporarily admitted to the local hospital until the lesions crusted and the upper respiratory tract swab PCR test was negative and then discharged.
Of particular note are the family monkeypox clusters that occurred in 2021. The family (father, mother and four children under 10) came to the UK from Nigeria. During a 10-day period of mandatory self-isolation with COVID-19, the father (patient 5 [2021]) developed a progressive vesicular rash, which he believed was caused by chickenpox. After the quarantine period ended, he visited the local emergency room and was admitted to the regional HCID center, where he was diagnosed with monkeypox by vesicular fluid PCR testing.
When the youngest child subsequently developed fever and blisters, the whole family was admitted to the same HCID ward. The three older siblings were grouped with their father, who was thought to be non-infectious at the time, and the three children and their father were discharged after blood and upper respiratory tract swabs were taken and PCR was negative for monkeypox virus. Completed 21-day quarantine at home.
The mother of the family requested to be hospitalized and continued to care for her daughter (Patient 6 [2021]). After the child's monkeypox was confirmed by PCR testing of lesion swabs, medical personnel considered taking tecovirimat for treatment, but considering that the drug was not approved for use in children at the time, and it was not suitable for children weighing less than 13 kg. The patient did not have a standardized dose and had only previously been given it in a child with smallpox infection, so it was not used. After the 20th day of onset, the PCR test for monkeypox virus in the upper respiratory tract swab of patient 6 was negative.
However, on day 14 of the onset of patient 6 (2021), the mother (patient 7 [2021]) developed headache, pharyngitis, and chest vesicles, and the monkeypox DNA PCR test was positive. In the mother's isolation, the team decided to offer a 2-week course of oral tecovirimat (600 mg twice daily). The aim of treatment is to prevent complications and shorten the length of hospital stay. Samples from the patient's blood and upper respiratory tract were PCR negative 48 hours after taking tecovirimat and remained negative at 72 hours. No new lesions appeared 24 hours after tecovirimat treatment.
The choice of the two drugs during treatment is undoubtedly the most concerning. In this regard, the researchers commented, "When the first monkeypox patients were diagnosed in the UK in 2018, brincidofovir and tecovirimat were not licensed for the treatment of monkeypox. Brincidofovir was selected in 2018 because of the reuse of supplies already in local clinical trials by medical personnel in an emergency situation. Given the small number of people involved, it is difficult to infer bricindofovir The relationship between fovir treatment and disease course. Although in several samples, monkeypox virus PCR cycle thresholds were transiently decreased after patients took the drug, these improvements were not durable and were also seen between patients. Not consistent. We do not know whether administration earlier in the disease course or with a different dosing regimen would have resulted in more beneficial clinical outcomes with brincidofovir. All 3 patients experienced post-treatment Disturbance of liver enzymes, which directly led to the decision to reduce the duration of medication. However, in the treatment of prairie dogs with confirmed monkeypox infection, brincidofovir had a modest survival benefit (29% vs 14%), and reduced viral titers in end organs."
"The use of tecovirimat in the treatment of patient 7 (2021) is based on the evidence of efficacy of the drug in animal models of orthopoxvirus infection and the drug's good tolerance in humans. Physicians hope This treatment regimen prevents disease progression or shortens the length of hospital stay. Treatment begins shortly after skin lesions develop. Tecoviride compared to patients not taking the drug or those treated with brincidofovir The clinical and virological responses of (tecovirimat) were faster and time-correlated, however, we were not sure whether this was the result of tecovirimat treatment. In the treatment of smallpox-infected macaques, compared with placebo, Tecovirimat was also effective in reducing the number of lesions in the blood and upper respiratory tract and shortening the duration of PCR positivity. Available data suggest that a 5-day course of tecovirimat is sufficient to produce clinical responses, whereas A 2-week course of treatment produces humoral immunity and durable virus clearance.
In addition, the researchers emphasized in the paper that great attention should be paid to children infected with monkeypox. Based on historical data, monkeypox infection in children is more likely to be severely ill than adults, and is also more associated with death, they said. Patient 6 (2021) is the first pediatric patient with monkeypox reported outside Africa since 2003 and the first pediatric patient managed by the UK HCID network. During treatment, a paediatric team from the UK Department of Child Health and Social Development provided on-site care in an adult isolation ward, addressing the challenges of managing a young child and an adult in isolation. The pediatric patient required careful multidisciplinary and interprofessional coordination, collaboration, and communication, and fortunately, this pediatric patient had a milder disease course.
On the viral dynamics of human monkeypox infection, the researchers stated, "In previous monkeypox cases and outbreaks, patients were thought to be infectious until all lesions had crusted over. In these seven patients' disease course follow-up , we observed trajectories of positive PCR in patient blood and upper respiratory tract swabs, similar to non-human primate models of monkeypox and smallpox infection. It took at least 3 weeks for viral DNA shedding in upper respiratory swabs from the first 3 patients Time. The infectivity of patients at the time of upper respiratory tract swab positive and skin crusting lesions remains undetermined and is an important area for future research with immediate practical implications for health care resource use, safe patient discharge, and prevention of transmission.
In addition, for the recurrence of patient 4 (2019), the researchers believe that this is related to mild, short-term clinical disease and transient shedding of monkeypox virus DNA. The temporal correlation between sexual intercourse, increased inguinal lymph nodes, and rash recurrence may suggest that the genitals may act as a reservoir for monkeypox virus. But the theory warrants dedicated study in larger patient samples.
The paper also mentions, "All patients were young, had no pre-existing complications, and none had been vaccinated against smallpox prior to exposure to the virus. Nonetheless, most patients had a relatively mild course of disease, which is consistent with the monkeypox virus in West Africa. The infection is consistent with bronchial infection. Depressed mood is more common.”
None of the patients experienced any serious complications common to monkeypox, such as pneumonia or superimposed bacterial sepsis. Patient 2 (2018) represents the first adult patient report of a deep tissue monkeypox abscess. Neither of the patient's two abscesses directly communicated with the superficial skin lesions, and the thigh abscess was found by ultrasonography about 2 weeks after diagnosis, and the PCR cycle threshold for monkeypox virus in the abscess fluid was low.
Finally, the investigators acknowledge some limitations of the study, namely the observational nature and the small number of cases analyzed. The researchers were also unable to confirm a positive monkeypox PCR test with laboratory cultures of the virus, meaning that the circulating infectious virus could not be confirmed.
"The cases reported in our study, as well as the recent outbreak, highlight the importance of maintaining a global collaborative network that we need to manage sporadic outbreaks of high-consequence pathogens such as monkeypox." Liverpool, UK Dr Nick Price, University NHS Trust and one of the authors of the paper, said: "The cases we observed were extremely challenging to manage and difficult to manage even in a high-income setting like the UK. As international travel returns to pre-pandemic levels, public health officials and health care workers around the world must remain vigilant about the potential for new cases of monkeypox."
Attached: Chart



Related Posts
0 Comments
Write A Comments