
Between 5 April and 26 May 2022, 650 suspected cases of acute hepatitis of unknown etiology in children were reported to WHO from 33 countries in 5 WHO regions. The cause of this severe acute hepatitis is unknown and is under investigation.
On May 27, local time, the WHO issued the latest disease notification. The majority (n=374, 58%) of the above 650 cases were from the WHO European Region, with 222 (34%) cases in the UK alone. In addition, probable cases and Cases to be classified.
Of concern, out of 650 cases, at least 38 (6%) children required transplantation, and nine (1%) deaths have been reported to WHO.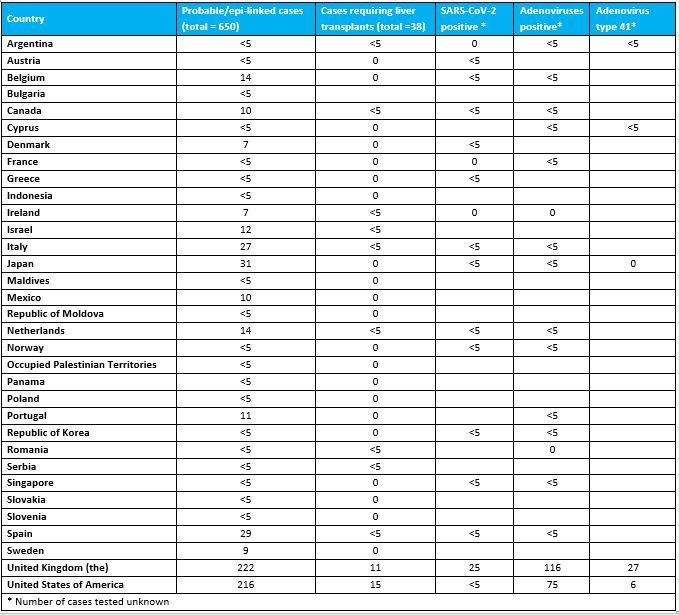
Overall, of 181 specimens tested for adenovirus, 110 (60.8%) tested positive. Whole blood samples had the highest positive rate of 69.5%. Regarding the detection of adenovirus, Isabella Eckerle, head of the Center for Emerging Infectious Diseases at the University of Geneva in Switzerland, had previously questioned that even if adenovirus was detected in the blood samples proposed by the British Health Security Agency, there was a problem that the viral load in the blood was very low. In some cases, the patient's adenoviral DNA was only present in whole blood and not in plasma. "If acute hepatitis is due to adenovirus, the virus replicating in the liver should be everywhere: in plasma, serum, biopsies, not just whole blood in most children."
Regarding the detection of SARS-CoV-2 infection, of the 188 SARS-CoV-2 PCR tests, 23 (12.2%) tested positive. It should be noted that currently only 26 of these cases worldwide have been serologically tested for SARS-CoV-2, of which 19 (73.1%) were positive. In fact, nucleic acid testing can only determine whether the child was infected with the new crown at the time, and serological testing is required to see whether the child has been infected with the new crown in the past.
Additionally, of the 63 cases with COVID-19 vaccination data, 53 (84.1%) were not vaccinated.
For these cases of unexplained childhood hepatitis reported to WHO since April, the etiology is unknown. Among them, the earliest reported cases and the most reported cases so far are the United Kingdom. The United Kingdom Health and Safety Agency (UKHSA) has proposed five "hypotheses", the first of which is caused by human adenovirus infection, which has also triggered extensive discussions. and controversy.
The WHO mentioned that adenovirus was found in 75% of cases tested in the UK, but data from other countries were incomplete. Of the small number of samples that have been typed to date, most have been identified as adenovirus type 41. In addition, metagenomes of liver and blood samples revealed that adenovirus-associated virus 2 (AAV-2) was also found in a small number of cases in the UK. However, WHO cautioned that many cases did not have appropriate samples taken, highlighting the importance of proper sampling (whole blood) to further determine the type of adenovirus detected. Furthermore, adenovirus type 41 infection has not previously been associated with this clinical presentation in otherwise healthy children.
While adenovirus is a plausible hypothesis for pathogenesis, further research into the pathogen is underway, WHO said. Adenovirus infection does not fully explain the more severe clinical symptoms observed in these cases, as it usually causes only mild, self-limited gastrointestinal or respiratory infections in young children.
WHO concluded that there are many possible pathogenic mechanisms to be further studied, including: during the COVID-19 pandemic, lower levels of adenovirus transmission increased susceptibility in young children; a new adenovirus may emerge; Complications of SARS-CoV-2 co-infection or previous SARS-CoV-2 infection, leading to superantigen-mediated activation of immune cells, etc. But it also stressed that hypotheses about the side effects of COVID-19 vaccines are currently not supported because most affected children are not vaccinated with these vaccines.
WHO has attached more information at the end of this latest update, which includes Professor Petter Brodin, Department of Immunology and Inflammation, Imperial College London, and Petter Brodin, Department of Paediatrics, Infectious Diseases and Immunology, Cedars Sinai Medical Center, Los Angeles, California, USA. Moshe Arditi previously published a communication article in The Lancet Gastroenterology & Hepatology, an international authoritative academic journal. This high-profile article explores that unexplained hepatitis in these children may be associated with the superantigen of the new coronavirus. The so-called super antigen (SAg) is a kind of substance that can activate a large number of T cell clones and generate a strong immune response with only a very low concentration. Compared with ordinary antigens, superantigens do not require conventional intracellular antigen presentation and have no MHC restriction.
In addition, two Chinese scholars also recently published a genome comparison analysis study on the preprinted website bioRxiv, proposing new ideas: the preliminary results show a possibility that the infection of the new coronavirus with the ORF1ab^VVVNASN mutation characteristics The strain may elicit autoimmune T-cell responses in patients via 'epitope mimicry' and has been associated with outbreaks of unknown hepatitis.
It is worth noting that the WHO also pointed out in this latest notification that the apparent association of these cases with adenovirus may be an accidental finding, which may be due to the intensification of laboratory testing for adenovirus and community transmission of adenovirus. level increases. The association of adenovirus remains to be further elucidated as adenovirus testing is expanded to other cases outside of Europe and the United States, and the results of a case-control study currently underway by the UK Health Security Agency are reported.
Dr Renu Bindra, senior medical adviser at the UK Health Security Agency, said on May 27, "Our investigations continue to suggest that adenovirus is linked, and we are continuing to study the association, as well as other possible factors, including previous infection with SARS- CoV-2 et al".
WHO recommends that samples of whole blood, serum, urine, stool, respiratory tract and liver biopsy (if available) should be collected in all cases meeting the definition. WHO is developing interim guidance and establishing a regional and global network of referral laboratories to support Member States in laboratory testing.
WHO emphasizes the urgent need to collect more information to assess the potential role of infections, including adenovirus and current and past SARS-CoV-2 infections, and to investigate other potential factors (other infections, toxins, drugs or other underlying disease).
WHO currently assesses the global risk as moderate. Mainly based on the following considerations:
The etiology of this severe acute hepatitis is unknown and is under investigation; compared with previously reported acute hepatitis of unknown etiology in children, this case was clinically severe and had a higher rate of progression to acute liver failure;
WHO currently has limited epidemiological, laboratory, histopathological and clinical information;
In some cases, the actual number of cases may be underestimated, in part due to the limited surveillance capacity available;
The source and mode of transmission of the potential pathogen have not been identified, so the possibility of further transmission cannot be fully assessed;
While there are no existing reports of healthcare-associated infections, human-to-human transmission cannot be ruled out as there have been some reports of epidemiologically relevant cases.
On May 27, local time, the WHO issued the latest disease notification. The majority (n=374, 58%) of the above 650 cases were from the WHO European Region, with 222 (34%) cases in the UK alone. In addition, probable cases and Cases to be classified.
Of concern, out of 650 cases, at least 38 (6%) children required transplantation, and nine (1%) deaths have been reported to WHO.

Probable cases reported by countries from 1 October 2021 to 26 May 2022.
According to the latest joint surveillance report by the WHO European Regional Office (EURO) and the European Centre for Disease Control and Prevention (ECDC) on cases from EU/EEA countries reported through the European Surveillance System (TESSy), as of 20 May, Three-quarters (75.4%) of cases were children under the age of 5; of 156 cases with hospitalization information, 22 (14.1%) were admitted to intensive care; of 117 patients, 14 (12%) received liver transplant.Overall, of 181 specimens tested for adenovirus, 110 (60.8%) tested positive. Whole blood samples had the highest positive rate of 69.5%. Regarding the detection of adenovirus, Isabella Eckerle, head of the Center for Emerging Infectious Diseases at the University of Geneva in Switzerland, had previously questioned that even if adenovirus was detected in the blood samples proposed by the British Health Security Agency, there was a problem that the viral load in the blood was very low. In some cases, the patient's adenoviral DNA was only present in whole blood and not in plasma. "If acute hepatitis is due to adenovirus, the virus replicating in the liver should be everywhere: in plasma, serum, biopsies, not just whole blood in most children."
Regarding the detection of SARS-CoV-2 infection, of the 188 SARS-CoV-2 PCR tests, 23 (12.2%) tested positive. It should be noted that currently only 26 of these cases worldwide have been serologically tested for SARS-CoV-2, of which 19 (73.1%) were positive. In fact, nucleic acid testing can only determine whether the child was infected with the new crown at the time, and serological testing is required to see whether the child has been infected with the new crown in the past.
Additionally, of the 63 cases with COVID-19 vaccination data, 53 (84.1%) were not vaccinated.
For these cases of unexplained childhood hepatitis reported to WHO since April, the etiology is unknown. Among them, the earliest reported cases and the most reported cases so far are the United Kingdom. The United Kingdom Health and Safety Agency (UKHSA) has proposed five "hypotheses", the first of which is caused by human adenovirus infection, which has also triggered extensive discussions. and controversy.
The WHO mentioned that adenovirus was found in 75% of cases tested in the UK, but data from other countries were incomplete. Of the small number of samples that have been typed to date, most have been identified as adenovirus type 41. In addition, metagenomes of liver and blood samples revealed that adenovirus-associated virus 2 (AAV-2) was also found in a small number of cases in the UK. However, WHO cautioned that many cases did not have appropriate samples taken, highlighting the importance of proper sampling (whole blood) to further determine the type of adenovirus detected. Furthermore, adenovirus type 41 infection has not previously been associated with this clinical presentation in otherwise healthy children.
While adenovirus is a plausible hypothesis for pathogenesis, further research into the pathogen is underway, WHO said. Adenovirus infection does not fully explain the more severe clinical symptoms observed in these cases, as it usually causes only mild, self-limited gastrointestinal or respiratory infections in young children.
WHO concluded that there are many possible pathogenic mechanisms to be further studied, including: during the COVID-19 pandemic, lower levels of adenovirus transmission increased susceptibility in young children; a new adenovirus may emerge; Complications of SARS-CoV-2 co-infection or previous SARS-CoV-2 infection, leading to superantigen-mediated activation of immune cells, etc. But it also stressed that hypotheses about the side effects of COVID-19 vaccines are currently not supported because most affected children are not vaccinated with these vaccines.
WHO has attached more information at the end of this latest update, which includes Professor Petter Brodin, Department of Immunology and Inflammation, Imperial College London, and Petter Brodin, Department of Paediatrics, Infectious Diseases and Immunology, Cedars Sinai Medical Center, Los Angeles, California, USA. Moshe Arditi previously published a communication article in The Lancet Gastroenterology & Hepatology, an international authoritative academic journal. This high-profile article explores that unexplained hepatitis in these children may be associated with the superantigen of the new coronavirus. The so-called super antigen (SAg) is a kind of substance that can activate a large number of T cell clones and generate a strong immune response with only a very low concentration. Compared with ordinary antigens, superantigens do not require conventional intracellular antigen presentation and have no MHC restriction.
In addition, two Chinese scholars also recently published a genome comparison analysis study on the preprinted website bioRxiv, proposing new ideas: the preliminary results show a possibility that the infection of the new coronavirus with the ORF1ab^VVVNASN mutation characteristics The strain may elicit autoimmune T-cell responses in patients via 'epitope mimicry' and has been associated with outbreaks of unknown hepatitis.
It is worth noting that the WHO also pointed out in this latest notification that the apparent association of these cases with adenovirus may be an accidental finding, which may be due to the intensification of laboratory testing for adenovirus and community transmission of adenovirus. level increases. The association of adenovirus remains to be further elucidated as adenovirus testing is expanded to other cases outside of Europe and the United States, and the results of a case-control study currently underway by the UK Health Security Agency are reported.
Dr Renu Bindra, senior medical adviser at the UK Health Security Agency, said on May 27, "Our investigations continue to suggest that adenovirus is linked, and we are continuing to study the association, as well as other possible factors, including previous infection with SARS- CoV-2 et al".
WHO recommends that samples of whole blood, serum, urine, stool, respiratory tract and liver biopsy (if available) should be collected in all cases meeting the definition. WHO is developing interim guidance and establishing a regional and global network of referral laboratories to support Member States in laboratory testing.
WHO emphasizes the urgent need to collect more information to assess the potential role of infections, including adenovirus and current and past SARS-CoV-2 infections, and to investigate other potential factors (other infections, toxins, drugs or other underlying disease).
WHO currently assesses the global risk as moderate. Mainly based on the following considerations:
The etiology of this severe acute hepatitis is unknown and is under investigation; compared with previously reported acute hepatitis of unknown etiology in children, this case was clinically severe and had a higher rate of progression to acute liver failure;
WHO currently has limited epidemiological, laboratory, histopathological and clinical information;
In some cases, the actual number of cases may be underestimated, in part due to the limited surveillance capacity available;
The source and mode of transmission of the potential pathogen have not been identified, so the possibility of further transmission cannot be fully assessed;
While there are no existing reports of healthcare-associated infections, human-to-human transmission cannot be ruled out as there have been some reports of epidemiologically relevant cases.
Related Posts
0 Comments
Write A Comments