Recently, the team of Zhao Jin from the School of Physics, University of Science and Technology of China found that the ultrafast charge transfer at the solid-molecular interface is strongly coupled with the quantum dynamics of protons, revealing the important role of nuclear quantum effects in the charge transfer process. 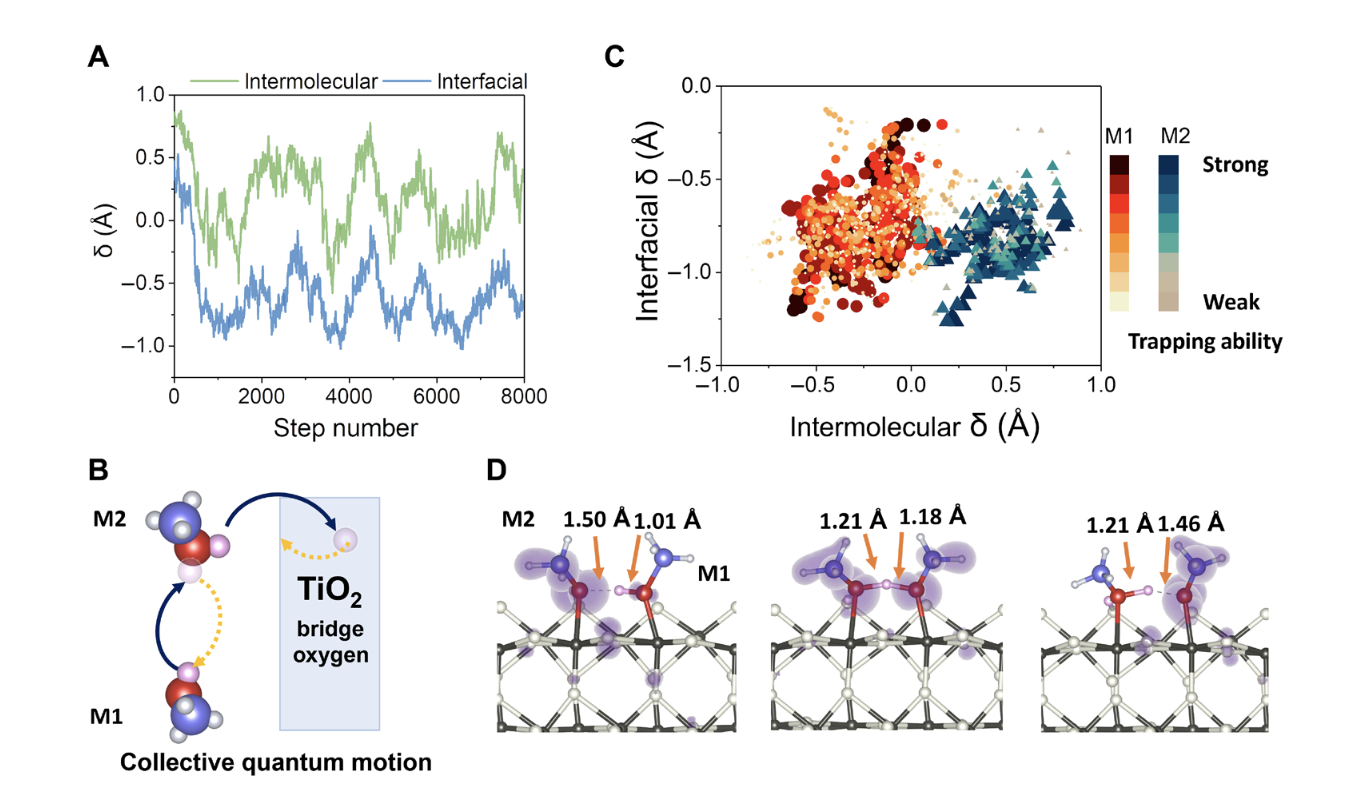

At many solid-molecular interfaces, a complex network of hydrogen bonds is formed between molecules, and protons are often transferred in such a network of hydrogen bonds. Therefore, charge transfer at the solid-molecular interface is often coupled with the motion of protons. In the process, scientists are faced with a complex quantum system that not only needs to understand the dynamic behavior of electrons, but also need to consider their coupling with protons. And the protons moving in the hydrogen bond network have their own nuclear quantum effects that cannot be ignored, which has become an unsolved complex problem in related fields.
This time, Zhao Jin's team cooperated with Li Xinzheng's team to use the first-principles excited state dynamics software Hefei-NAMD developed by Zhao Jin's research group to combine two cutting-edge calculation methods in the field of first-principles calculation, namely "diabatic". Molecular dynamics (NAMD)" combined with "path-integrated molecular dynamics (PIMD)" solves the aforementioned challenges.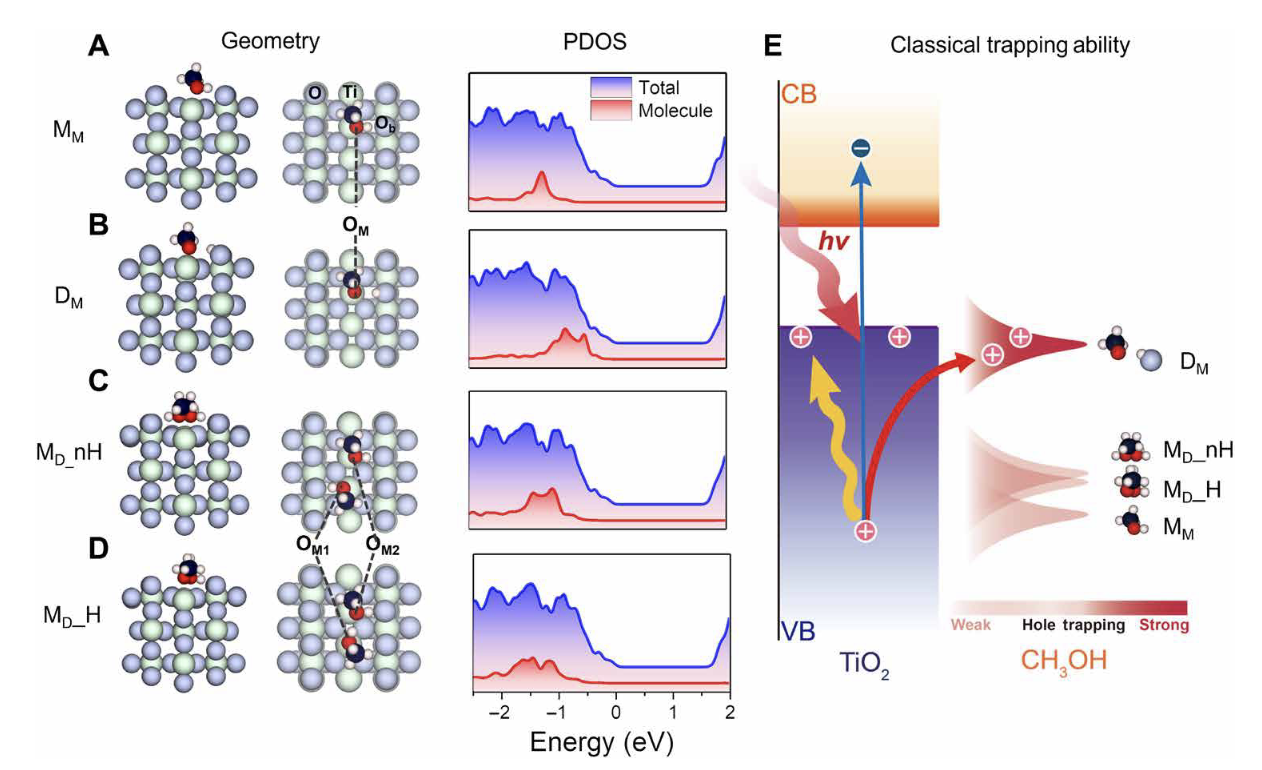
On the one hand, the aforementioned studies reveal the important role of the formation of hydrogen bond networks and nuclear quantum effects in the ultrafast charge transfer process at the molecular-solid interface. On the other hand, it provides new tools for studying the coupling of nuclear quantum dynamics and electron dynamics using first-principles calculations.

Proton-coupled charge transfer at the CH3OH/TiO2 (methanol/titanium dioxide) interface, picture from University of Science and Technology of China
The aforementioned research was carried out by the research team of Professor Zhao Jin from the School of Physics, University of Science and Technology of China, Hefei National Research Center for Microscale Matter Science, International Center for Quantum Design of Functional Materials (ICQD), Hefei National Laboratory, Wang Bing, Tan Shiliang, and Peking University Professor Li Xinzheng. Collaboration. The results were published in Science Advances.
Image via Science Advances
The solid-molecular interface is one of the most important prototype systems to study the solar energy conversion process, and the photo-excited carrier dynamics at the interface is one of the decisive factors to determine the solar energy conversion efficiency. In typical solar energy conversion processes such as photocatalysis and photovoltaics, photoexcitation generates electron-hole pairs in semiconductor materials, and these excited carriers are then transferred to molecules through solid-molecular interfaces.At many solid-molecular interfaces, a complex network of hydrogen bonds is formed between molecules, and protons are often transferred in such a network of hydrogen bonds. Therefore, charge transfer at the solid-molecular interface is often coupled with the motion of protons. In the process, scientists are faced with a complex quantum system that not only needs to understand the dynamic behavior of electrons, but also need to consider their coupling with protons. And the protons moving in the hydrogen bond network have their own nuclear quantum effects that cannot be ignored, which has become an unsolved complex problem in related fields.
This time, Zhao Jin's team cooperated with Li Xinzheng's team to use the first-principles excited state dynamics software Hefei-NAMD developed by Zhao Jin's research group to combine two cutting-edge calculation methods in the field of first-principles calculation, namely "diabatic". Molecular dynamics (NAMD)" combined with "path-integrated molecular dynamics (PIMD)" solves the aforementioned challenges.

Geometric configuration and electronic structure of CH3OH/TiO2 (methanol/titanium dioxide) surface, picture from University of Science and Technology of China
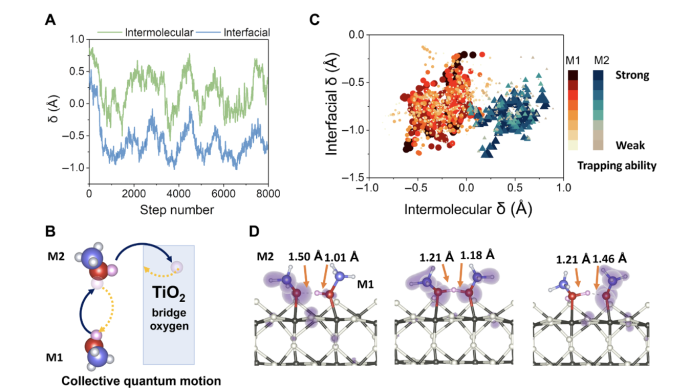
They used non-adiabatic molecular dynamics to deal with the electron dynamics part, and a ring-polymerization molecular dynamics (RPMD) method based on path-integral theory to deal with nuclear quantum effects. Based on this scheme, the team studied the kinetics of hole transfer at the CH3OH/TiO2 (methanol/titanium dioxide) interface, and found that when methanol adsorbed on the surface of titanium dioxide forms a hydrogen bond network, protons will frequently transfer in the network, and these proton motions have obvious quantized behavior. Therefore, the trapping ability of the adsorbed methanol molecules for excited state holes is significantly enhanced due to the quantized motion of protons, thereby enhancing the efficiency of photochemical reactions. This conclusion is found in Tan Shijing and Wang Bing's scanning tunneling microscope (STM) experiments.On the one hand, the aforementioned studies reveal the important role of the formation of hydrogen bond networks and nuclear quantum effects in the ultrafast charge transfer process at the molecular-solid interface. On the other hand, it provides new tools for studying the coupling of nuclear quantum dynamics and electron dynamics using first-principles calculations.
Related Posts
Write A Comments
Amazing a good deal of wonderful material! website Kudos! Ample stuff. casino en ligne Truly lots of helpful info! casino en ligne Kudos! Good information! casino en ligne Wow lots of beneficial information! casino en ligne You have made your point! casino en ligne Thanks. Good stuff! casino en ligne Point very well used.. casino en ligne Regards! A good amount of posts. casino en ligne Many thanks. I enjoy this! casino en ligne