① Schematic diagram of the morphology of human mature red blood cells.
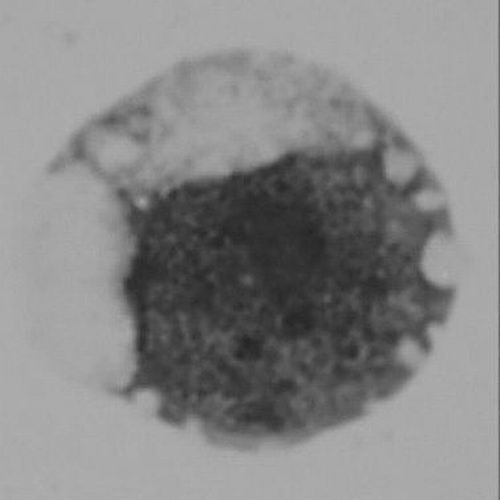
②The early stage of terminal development of human erythrocytes observed under light microscope.
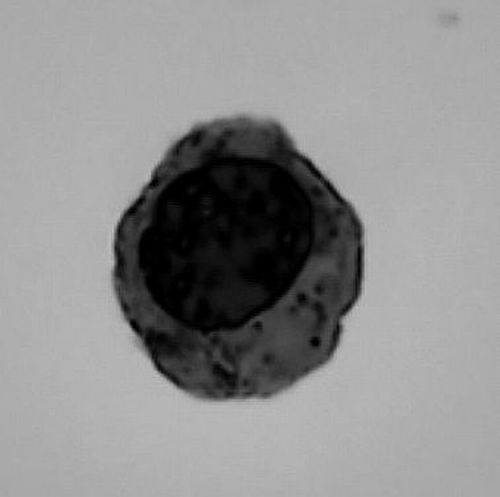
③Late stage of terminal development of human erythrocytes observed under light microscope. Photo provided by the interviewee
As the most numerous cell in the body, red blood cells transport oxygen and carbon dioxide through blood vessels large and small, sometimes having to fold themselves to pass through those tiny capillaries. This causes the red blood cells to "give up" their nucleus and work at full capacity in the body for about 120 days.
Until now, scientists have not fully understood how red blood cells compress the nucleus and "push" it out of the cell.
On March 14, the research group of Li Xiangying, a professor at the School of Life Sciences of Peking University and the Peking University-Tsinghua Joint Center for Life Sciences, reported for the first time in "Nature Structural and Molecular Biology" the large size of heterochromatin in the process of terminal differentiation of human erythrocytes. Scale remodeling may be the main contributor to chromatin compaction, and large-scale collapse of chromatin topology-associated domains occurs, and the transcription factor GATA1 participates in the maintenance of undisintegrated chromatin topology-associated domains. This will lay a theoretical foundation for understanding and promoting research on red blood cell-related diseases.
"Denucleated" red blood cells
Red blood cells are the most active self-renewal system in the human body. A healthy human body needs to renew about 2 billion red blood cells every day. Li Xiangying, who has long been concerned with the research of red blood cell development and related diseases, said in an interview with "China Science Daily" that defects in the red blood cell development process can lead to anemia or many diseases of red blood cell dysplasia.
The process of red blood cell development can be divided into several stages. Mature red blood cells are usually biconcave discs and have no nucleus. Therefore, during their development, precursor cells need to undergo processes such as chromatin compaction and nuclear polarization. Mammalian red blood cells eventually undergo "denucleation" A critical step to produce red blood cells without nuclei.
Li Xiangying introduced that scientists have long observed that red blood cells become smaller in size during the maturation process, and as their chromatin is gradually compressed, the nuclear capacity is finally reduced to 1/3 of its original size. However, the expression of genes related to red blood cell function such as the hemoglobin gene still needs to be maintained throughout the process. "Whether there are certain procedural rules and mechanisms for the chromatin compression process in red blood cell development, and how to maintain gene expression in a highly compressed state of chromatin, have always been unresolved issues." Li Xiangying said.
Previous studies have shown that several epigenetic factors can regulate this process. But no studies have observed the fine-grained process of chromatin compaction at the genome-wide level. More importantly, there is no genome-wide data to find the rules and principles of high-frequency loci or trend changes of chromatin compaction.
Programmatic compaction of chromatin
Li Dong, the first author of the paper and a postdoctoral fellow at the School of Life Sciences of Peking University, introduced that there are two different types of chromatin in the chromosome structure—heterochromatin and euchromatin. Euchromatin refers to the looser, denser gene-dense, more actively transcribed regions of chromosomes. Heterochromatin refers to densely compressed regions of chromosomes that contain relatively few genes, but they play an important role in the process of cell differentiation, helping to determine the specificity and function of cells.
Chromatin topology-associated domains are clusters of regions within chromatin that are spatially adjacent and share a common regulatory mechanism. Chromatin topology-associated domains can contain multiple genes and their regulatory elements and play important roles in gene regulation. Studies have shown that the spatial organization of chromatin has an important impact on the regulation of gene expression, and chromatin topology-related domains, as a structural feature of the genome, can coordinate and regulate the expression and interaction of genes in it.
"Our study found that heterochromatin regions and euchromatin regions exhibit different compaction characteristics. As the main players in the compaction process, heterochromatin regions form interactions between compartments; while in euchromatin regions, accompanied by With global chromatin compression, some regions of chromatin topology-related domains disintegrate, but some regions remain intact and play gene regulatory functions." Said Wu Fan, the first author of the paper and a doctoral student at the School of Life Sciences, Peking University .
Emery Bresnick, professor in the Department of Cell and Regenerative Biology at the University of Wisconsin-Madison and director of the Wisconsin Blood Cancer Institute, said the results were surprising—a large number (about 60%) of chromatin topology-associated domains were compacted throughout chromatin. The process is disrupted and disintegrated, but the specific domain where the erythroid gene group is located remains intact until the end of cell differentiation. The erythroid gene cluster resides in specific domains containing active chromatin marks as well as the essential erythroid transcription factor GATA1, which should be important determinants of this selectivity.
Li Xiangying explained that GATA1 is an important transcription factor in the development of red blood cells. It mainly regulates the expression of red blood cell-related genes and promotes red blood cell development by binding to promoters and regulating enhancer-promoter interactions.
However, the function of GATA1 in the chromatin topology-associated domains that maintain erythrocyte development has not been reported. "In our study, knockout of GATA1 resulted in the disappearance of these maintained chromatin topology-associated domains; we also isolated nuclei that eventually protruded during development and found that even in nuclei that had detached, chromatin remained homogeneous." Similar chromatin topology-related domains before denucleation." Li Xiangying said.
Laying the theoretical foundation for in vitro hematopoiesis
"The research of Li Xiangying's team focuses on an important developmental mechanism—the high-level chromatin structural changes in the process of chromatin condensation in the development of human red blood cells." Emery Bresnick said that this process involves compressing 70% to 80% of the nucleus volume, while denucleation Preserve and build specific new functionality.
Li Xiangying's team established a primary human erythroid differentiation system to solve this problem, revealed the basic principles and characteristics of chromatin compaction in erythropoiesis, and provided a powerful model for studying chromatin high-level structural changes in development and disease.
"The chromatin compression before red blood cell denucleation and the final denucleation process have always been the research focus of the regulation mechanism of red blood cell development, and the key to improving the efficiency of hematopoiesis in vitro." Professor An Xiuli, director of the New York Blood Center Biology Laboratory, believes that this This work provides strong data support and lays a theoretical foundation for future research on the regulation mechanism of chromatin structure in the process of erythrocyte terminal differentiation.
An Xiuli said that on the basis of this work, it is worthwhile to clarify the molecular mechanism of GATA1 maintaining the structure of chromatin topology-related domains, to find new regulatory targets for erythrocyte terminal differentiation and to improve the efficiency of in vitro hematopoiesis, especially denucleation. important direction.
"Next, we want to further study the regulators of red blood cell denucleation process, so as to fully understand the whole process of red blood cell formation. This research will provide some new ideas for in vitro hematopoiesis or treatment of red blood cell dysplasia, anemia and other diseases." Li Xiangying said.
(Original title "Exploring the "Folding Method" of Red Blood Cells Paving the Way for In Vitro Hematopoiesis")
Related paper information: https://doi.org/10.1038/s41594-023-00939-3