

There are plastic garbage floating in some rivers, plastic garbage floating in the ocean, and even plastic garbage in the soil of some fields.
Is plastic the best invention, or the worst?
It is convenient and durable, and was once hailed as a symbol of modern industry, but it has "mixed reputations" and even became a disaster: plastic waste is floating in some rivers, plastic waste is floating in the ocean, and even plastic waste remains in the soil of some fields.
The reason, Jiang Xuefeng, a professor and doctoral supervisor at the School of Chemistry and Molecular Engineering of East China Normal University, believes that it is because the life cycle of plastics has not achieved a closed loop - it is difficult to degrade the cycle.
Once produced, plastic seems to exist forever, eventually either staying in the soil, or flowing into the ocean, or even being "shredded" into tiny microplastics, which enter the human body through food or water.
Recently, Jiang Xuefeng's research group provided a solution: photolysis - using light to degrade plastics at room temperature and pressure. The catalysts used in this process are sourced from the ocean.

Jiang Xuefeng, professor and doctoral supervisor of the School of Chemistry and Molecular Engineering, East China Normal University.
On July 29, related papers were officially published in Science Bulletin. The journal is an interdisciplinary scientific journal co-sponsored by the Chinese Academy of Sciences and the National Natural Science Foundation of China in 1950, published bimonthly.
The title of the paper is "Degradation of plastic wastes to commercial chemicals and monomers under visible light." Jiang Xuefeng is the corresponding author of the paper.
Catalysts extracted from seawater
Jiang Xuefeng told The Paper that through a large number of experiments, the team he led found that using uranyl ions as a catalyst and using light as an energy source can degrade common plastic waste. The whole process is environmentally friendly, energy-saving and efficient.
Is uranium 238 radioactive?
Jiang Xuefeng told The Paper that uranium 235 is radioactive, and uranium 238 is almost non-radioactive, which is very safe. There is a large amount of uranium in seawater, and the technology of extracting uranium from seawater has been widely used all over the world. "So people don't have to worry too much about safety and storage issues." Jiang Xuefeng said.
Jiang Xuefeng said that in the future, sunlight can be used for photodegradation. He explained, "The maximum absorption position of uranium 238 for light is 460 nanometers. Sunlight is visible light, including light between 400 and 800 nanometers. Therefore, using sunlight is no problem."
In experiments so far, they have been able to successfully degrade nine common plastics into raw materials for chemical products such as benzoic acid and terephthalic acid under normal temperature and pressure. "It can be used to make plastics, to make new and better plastics; it may also be turned into pharmaceutical intermediates and used in medicine; it may also be used in flavors and fragrances, materials, etc." Jiang Xuefeng said.
This technology is mild, broad-spectrum, and compatible with water and oxygen. Jiang Xuefeng explained, "Currently, one of the processes for plastic waste treatment is sorting and cleaning in the early stage. Only when it is very dry and pure can it be processed. However, the plastic waste we usually see will contain pigments, adhesives, and water stains. There are even stains on it. Our photodegradation, the bottle does not need to be cleaned, because it does not matter if there is water in it, the label does not need to be removed, and the pigment or adhesive foaming agent does not affect it, and it can still be degraded. Our work hopes to make the plastic Degradation promotes practicality and authenticity, and realizes the degradation of complex real scenes. And we have also realized the mixed degradation of five kinds of plastics. Let the plastic waste in the garbage dump become valuable, reusable basic high-purity raw material."
Using uranium nitrate hexahydrate as photosensitizer, polystyrene can obtain benzoic acid with 30% separation yield under 460nm blue light irradiation; polystyrene foam can be degraded into benzoic acid with 40% yield; high-impact polystyrene (HIPS) could be degraded to benzoic acid in 32% yield. Kilo-scale real polyethylene terephthalate (PET) bottles (unwashed, containing water, pigments, adhesives, additives) were able to degrade to terephthalic acid with 88% yield within 2 days.
In order to achieve application scale-up, the authors also designed and developed a novel continuous streamer reaction device for PET degradation, which has a 54,800-fold increase in efficiency compared with tube operation.
In the future, they will further expand the scope of experiments and develop suitable large-scale devices.
From elemental sulfur to plastic degradation
How did you come up with the idea of using uranium to catalyze the photodegradation of plastics?
Jiang Xuefeng said, "We are a scientific research group focusing on the research of sulfur chemistry. In the process of studying the precise control of the multivalent state of sulfur and the adjustment of the oxidation state of sulfur, we want to find a green and energy-saving method. We want to use the sun to Light direct sulfur oxidation. Because we want to avoid the high temperature, high pressure and high emissions that often occur in chemical production. We want to use a green way to oxidize sulfur. We found uranium and used it to oxidize sulfur, which can be oxidized from sulfide to sulfoxide , and can also be oxidized to sulfone to achieve precise regulation.”
Uranium plays a unique role in the overall oxidation process, he said. "Since it can oxidize sulfur so precisely, and it is so gentle and environmentally friendly, why not use it to oxidize and cut plastics? Because after oxidation, the chemical bonds will be cut, but it will not be completely shredded (increase carbon emissions), it can be accurately degraded. Let’s try it, and it really can oxidize, cut, and degrade plastic in a controlled manner.”
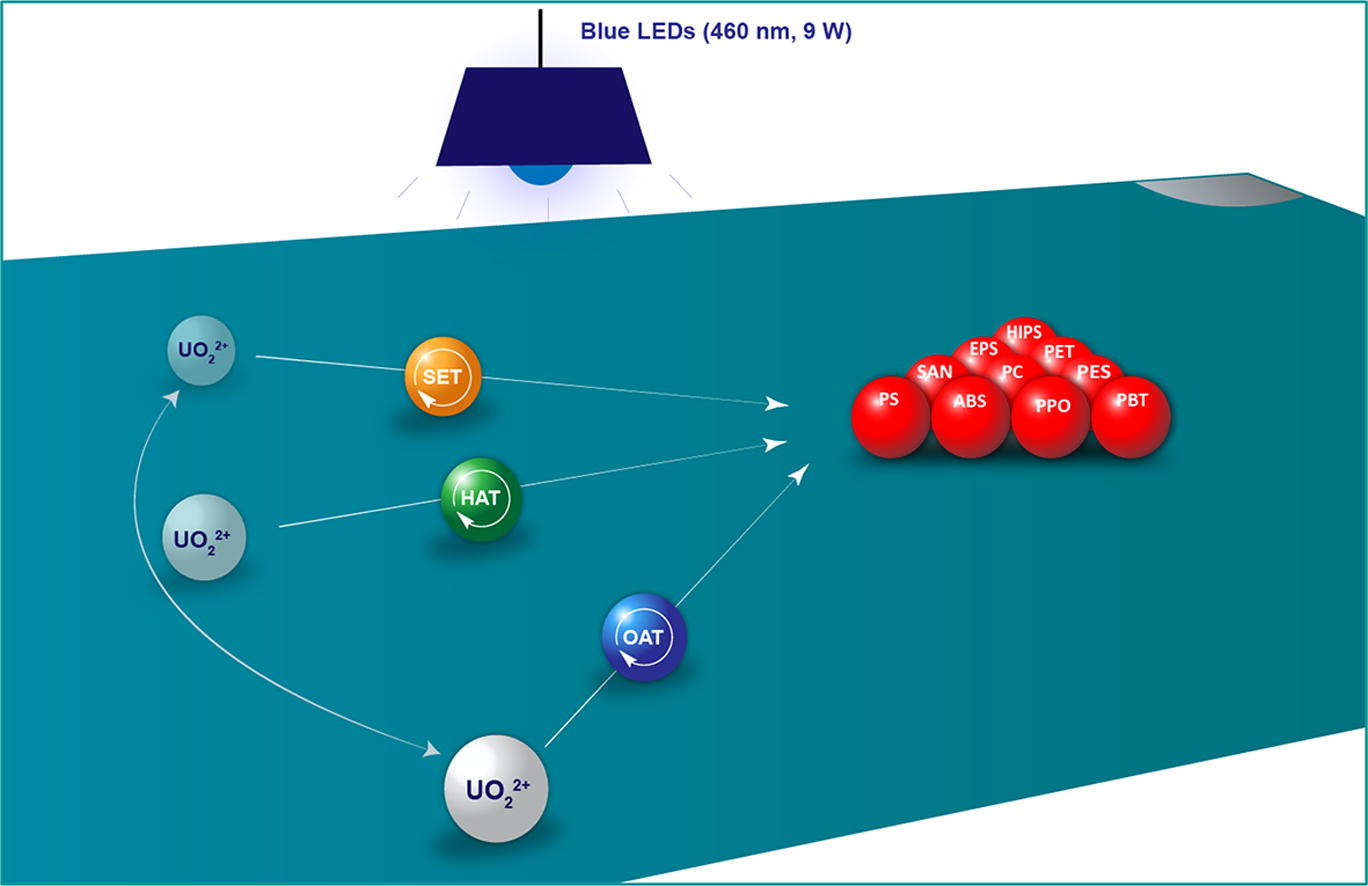
Schematic diagram: Under the action of a catalyst containing uranium 238, plastic is degraded under light.
"Originally, the natural decomposition of these plastics would take decades or even hundreds of years. We use this method to decompose them within a day or two, and decompose them to obtain very useful substances, such as benzoic acid, terephthalic acid, etc. High value-added fine chemicals." Jiang Xuefeng said.
Closed loop to the future
Jiang Xuefeng told The Paper that although the white pollution caused by plastic bags is indeed a headache for people, everyone has to admit that plastic bags are so light and practical that we humans will not give up eating because of choking. The crux of the problem lies in how to dispose, recycle, degrade, and use high-value materials after use.
"We are considering circular economy and closed-loop thinking economically, and we must also consider closed-loop and circular thinking scientifically. How to achieve closed-loop, that is, how to draw another semicircle, is the meaning of plastic degradation." "I think The most important thing about closed-loop thinking is that it cannot be discharged to the outside arbitrarily.” “The essence of abandonment is that it loses its value. But when it is valuable, whether it is an individual or a company, when new profits are generated, then it will be There will be a driving force for automatic collection and processing, and at this time our entire social development will be able to form a closed-loop sustainable development system.”
"Before, plastics were built with small molecule monomers, which provided convenience and convenience to humans; after use, they should not be thrown away in nature, and should not be burned to emit carbon dioxide. We should think about how to use low-energy It can be changed back to its original state at a low cost and low cost. In this way, as long as such carbon utilization can form a complete closed loop, we humans can live in harmony with nature, and at the same time meet more material needs and Production demand." Jiang Xuefeng said.
Attached paper link:
https://www.sciencedirect.com/science/article/abs/pii/S2095927323004073?via%3Dihub
