Recently, the famous neuroscientist Berislav V. Zlokovic of the University of Southern California (USC) has been accused of falsifying both "academic research papers" and "clinical trial data" and is undergoing internal review by the school.
Zlokovic has won the Potam Gold Medal, the "Nobel Prize" in the field of Alzheimer's disease research, and is considered the founder of the blood-brain barrier research field.
It is worth noting that the clinical trial of 3K3A-APC, an anti-stroke candidate drug developed by Zlokovic, is ready for Phase 3 trials. This is a human drug trial involving 1,400 stroke patients and receiving up to $30 million in funding. Now, the trial program has been suspended by the National Institutes of Health (NIH).

Berislav V. Zlokovic Source: USC
A 113-page whistleblower document revealed questionable data from this high-profile clinical trial. The document was written by Matthew Schrag, a neuroscientist at Vanderbilt University in the United States, and was recently provided to Science magazine.
In addition, four former members of Zlokovic’s lab told Science anonymously that Zlokovic often forced them and other members of the lab to adjust data.
$30 million human drug trial
Zlokovic co-founded a biopharmaceutical company called ZZ Biotech and holds about 3% of the company. With support from ZZ Biotech, he directed the development of 3K3A-APC to treat brain cell death that may occur after stroke. Publicly released phase 2 clinical trial data showed that the drug treatment was effective and reduced minor, asymptomatic cerebral hemorrhages.

ZZ Biotech official website introduces 3K3A-APC. Source: ZZ Biotech official website
In 2022, the NIH allocated $30 million and decided to use 3K3A-APC in 1,400 patients shortly after stroke.
The reason why 3K3A-APC attracts so much attention is that the development of stroke treatment drugs is extremely difficult and has rarely been successful. Currently, the only approved stroke drug in the United States and Europe is tPA (tissue plasminogen activator), which can greatly reduce death and disability by clearing stroke blockages, but it can also cause dangerous brain hemorrhages.
ZZ Biotech claims that 3K3A-APC can help reduce cerebral hemorrhage caused by tPA and prevent brain cell death.
For years, scientists have tried to reduce the brain cell death, bleeding and inflammation that can occur after a stroke. Due to the potential of 3K3A-APC to address unmet medical needs, the U.S. Food and Drug Administration (FDA) granted the compound "Fast Track" designation, and it is even expected to receive "Accelerated Approval and Priority Review." ZZ Biotech has said new trials should begin within months.
The development of stroke treatment drugs is difficult not only because of the complexity of the disease itself, but also because of the strict clinical trial standards for stroke treatment set by the Stroke Treatment Academic Industry Roundtable (STAIR).
Coincidentally, in a 2013 paper published in the journal Stroke, Zlokovic and colleagues evaluated research on 3K3A-APC and found that it met all of STAIR's criteria. They also provide evidence that 3K3A-APC reduces the volume of brain tissue damaged by stroke and prevents some bleeding caused by tPA.
Now, all this is being questioned.
A 113-page reporting document
Regarding the results of the Phase 2 trial of 3K3A-APC, the whistleblower document provides "shocking" evidence: 6 of the 66 stroke patients who received 3K3A-APC died within the first week after treatment, while those in the placebo group One of the 44 stroke patients died within a week of treatment. Although mortality rates stabilized after a month, patients who received the drug experienced more disability and dependence at the end of the trial - 90 days after treatment.
During the same period, patients who received 3K3A-APC had significantly higher mortality and disability rates than those who received placebo. This means the drug may pose serious health risks and has no clear efficacy advantage.
“It really boggles my mind to see a fourfold increase in mortality within the first few days of taking the drug,” says Wade Smith, a neuroscientist at the University of California, San Francisco. After reading the report, Smith felt so disturbed that He couldn't sleep that night.
In the document, the whistleblower also highlighted concerns about images in 35 published research papers by Zlokovic's team, as well as data from two phase 2 trials of 3K3A-APC.
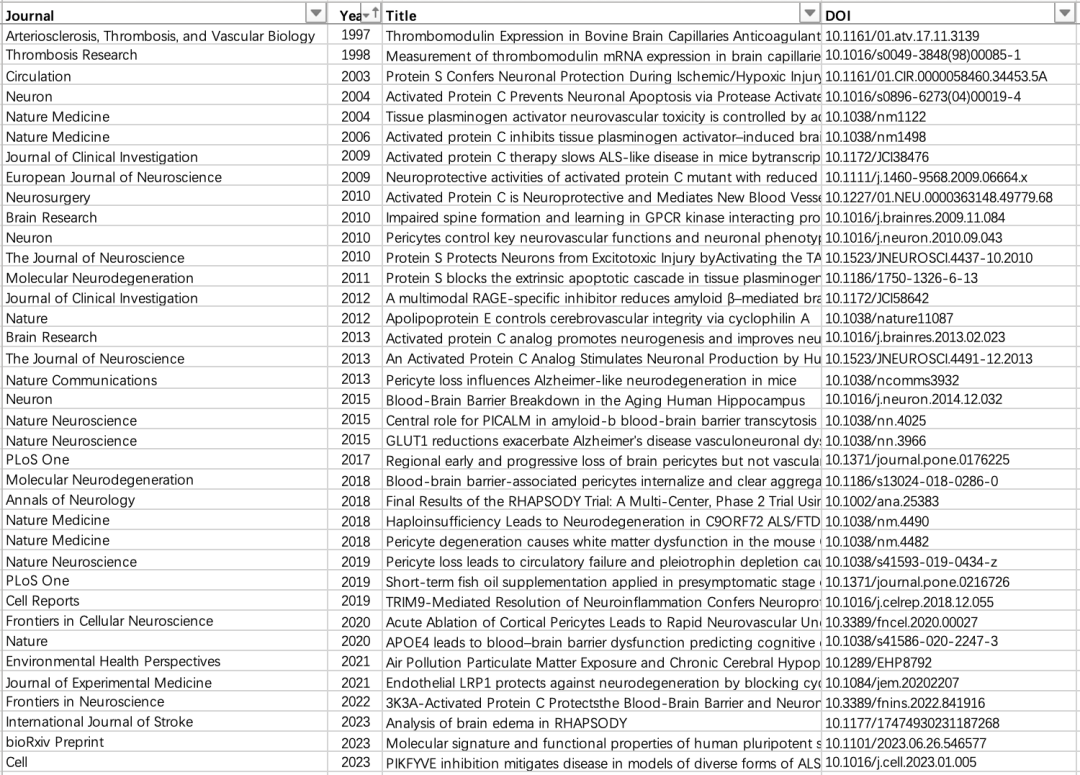
List of research papers included in the report file. Source: Report document
Berislav V. Zlokovic's name appears in all the above-mentioned publications, and he is the corresponding author in 29 of them (including the main report of the phase 2 trial). The earliest paper can be traced back to 1997, and most of the articles were published in top journals such as Nature and Cell.
The lead whistleblower, Matthew Schrag, hopes that the NIH will conduct a comprehensive review of the challenged papers after seeing the reporting documents. The introduction to the document states: "A number of articles appear to need to be retracted, and it is likely that this will also involve a range of grants."
A giant in the field of brain science
Zlokovic is an internationally recognized leader in Alzheimer's disease and stroke research. Diligent and prolific, he pioneered research on pericytes, cells that surround the brain's capillaries and help maintain the blood-brain barrier.
Zlokovic also linked the blood-brain barrier to Alzheimer's disease. Amyloid beta is one of the "culprits" in Alzheimer's disease, and the blood-brain barrier helps move amyloid beta out of the brain. This work earned him the $100,000 Bottom Gold Medal in 2009 from the American Academy of Neurology. The award honors researchers who have made pioneering and breakthrough achievements in the field of Pick's disease, Alzheimer's disease and related degenerative brain diseases.
Zlokovic is currently director of the USC Zilkha Institute of Neurogenetics. Under Zlokovic's leadership, the school's research institute has expanded to more than 30 laboratories and annual funding has increased more than 10 times. According to USC, the scale of his fundraising is staggering. At least $28 million has been raised from private sources in the past decade alone, and NIH grants to Zlokovic total $93 million.
Academic fraud, trying to hide it
Scientists and scientific detectives in related fields have conducted professional analysis and research on the papers listed in the reporting documents, including images and other signs of academic misconduct, and generally believe that the accusations against Zlokovic are not groundless.
Schrag recruited forensic image analyst Kevin Patrick, Columbia University neurobiologist Mu Yang, and the famous "academic counterfeiter" Elisabeth Bik to participate in the review of the work of Zlokovic's laboratory and published the relevant information on PubPeer. Commentary on Zlokovic's study.
Molecular biologist Mike Rossner and Bik agree that the whistleblower documents provide strong evidence of errors or misconduct in many of Zlokovic's papers.
For example, a 2013 study published in The Journal of Neuroscience showed that 3K3A-APC provides a range of protection for brain cells. The whistleblower said images of key Western blots in the study appeared to have been copied incorrectly and flipped horizontally.
A 2022 study published in Neuroscience showed that 3K3A-APC protects brain cells and the blood-brain barrier from stroke damage, but one of the key images appears to have been copied from a 2019 Nature Neuroscience paper in an unrelated field. Come.
In addition, the reporting documents also showed that Zlokovic had two papers published five years apart that seemed to use the same images to represent different experimental results.
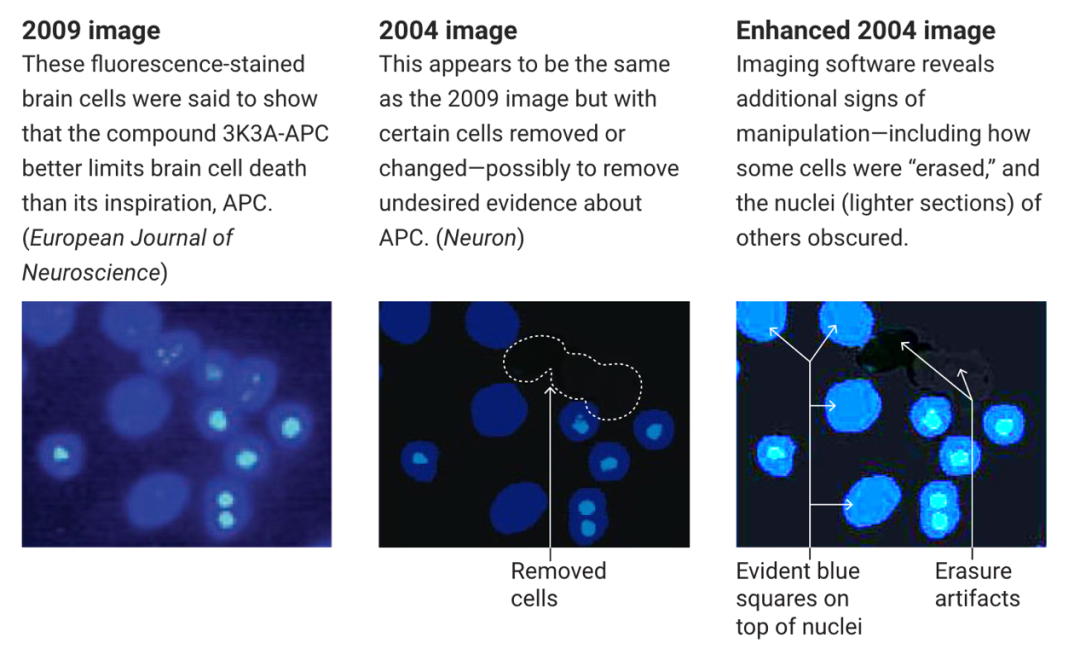
Comparison of research images of papers published 5 years apart
Chris Schaffer, a biomedical engineer at Cornell University in the United States, said this is an attempt to hide the signs that APC and 3K3A-APC are not actually protecting brain cells.
Four former lab members also accused Zlokovic of forcing them and others to manipulate data. They have all collaborated with Zlokovic for many years and published articles with him.
Members said their boss expected new data every week, or data that matched assumptions from his research. Zlokovic would often harshly criticize young scientists when they didn't get the experimental results he wanted.
One of the members said: "If you disagree with him, you will lose the lead authorship of the paper or project." Another said: "If the data does not seem to fit the hypothesis, we will not even dare to bring it to the experiment room meeting."
Is the field of neurological research in dire straits?
Data from Dimensions Analytics, an academic research database of British technology company Digital Science, shows that for decades, Zlokovic’s research on the impact of the blood-brain barrier and pericytes on stroke or Alzheimer’s disease has always been widely cited by other researchers and is a world leader. status.
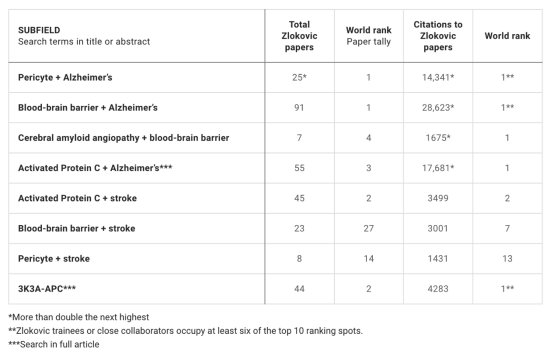
Data statistical results from the academic research database Dimensions Analytics.
In the reporting document, all of the questioned papers are related to Zlokovic's research on the blood-brain barrier, and the basic science papers (excluding the recently published preprint) have been cited more than 8,400 times.
On average, these papers are 27 times more influential than similar papers published in the same field or year, and have been cited in 49 patents by 30 companies, universities and foundations, according to Dimensions data.
The blood-brain barrier is becoming increasingly important in the study of cerebral amyloid angiopathy (CAA), Alzheimer's disease, stroke, and other neurological diseases. If Zlokovic's work is found guilty of misconduct, several subfields of neurological research may face a reckoning.
"We need to know clearly which of these findings are replicable and correct and which are just plain wrong," says Andreas Charidimou, a neuroscientist at Boston University.
Reference links:
https://www.science.org/content/article/misconduct-concerns-possible-drug-risks-should-stop-stroke-trial-whistleblowers-say
https://keck.usc.edu/faculty-search/berislav-v-zlokovic/
https://www.science.org/content/article/nih-puts-hold-30-million-trial-potential-stroke-drug