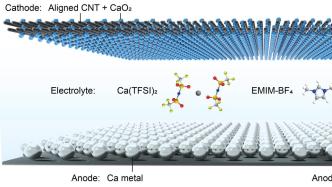
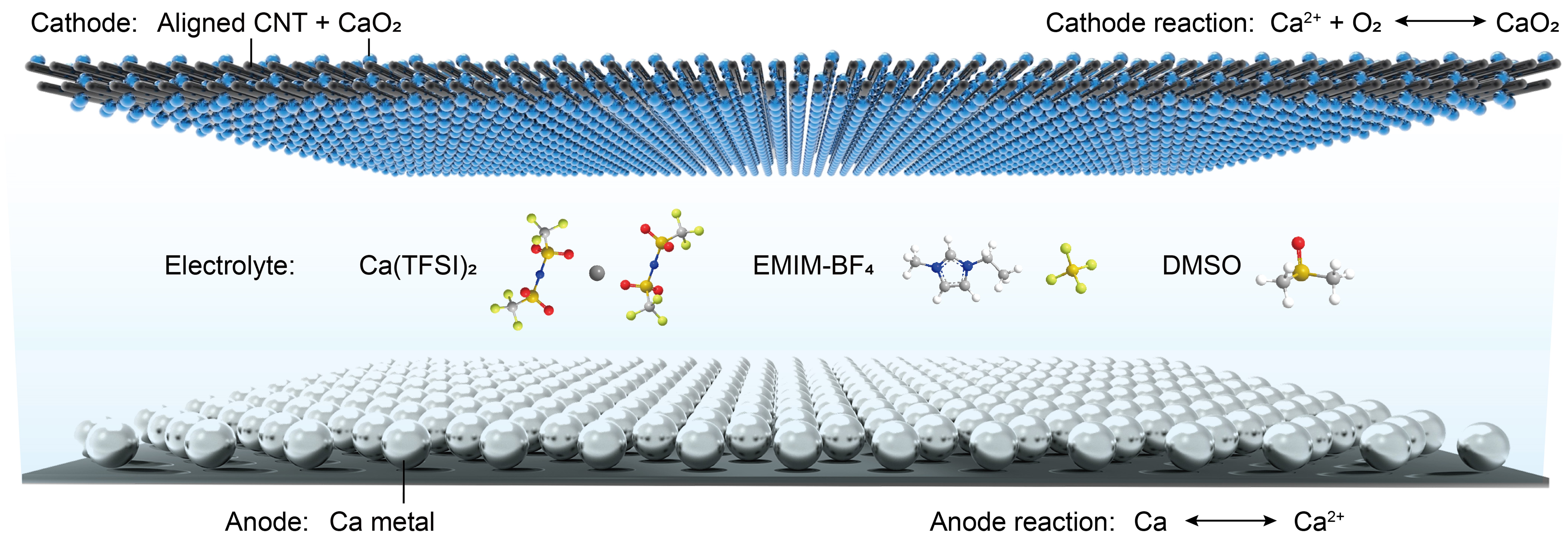
Schematic diagram of calcium-oxygen battery structure.
Fudan University has developed a new type of calcium-oxygen battery that can be charged and discharged at room temperature, operates stably for 700 cycles, and has the advantages of high flexibility, high safety, and lower cost. Such batteries can also be made into flexible fibers and woven into textile batteries for next-generation wearable systems.
On February 7, the relevant results were published online in the international academic journal Nature under the title "A rechargeable calcium-oxygen battery that operates at room temperature".
The aforementioned research was completed by the Peng Huisheng/Wang Bingjie team of Fudan University's Institute of Fiber Electronic Materials and Devices, Department of Polymer Science, Advanced Materials Laboratory, and State Key Laboratory of Polymer Molecular Engineering, together with collaborators such as Wang Yonggang, Zhou Haoshen, and Lu Jun.
Calcium or calcium compounds are oxidized to produce other oxides, which is the basic principle of calcium-oxygen batteries. In theory, calcium-oxygen batteries can be low-cost and high-capacity. Because it has the highest theoretical energy density among batteries based on metallic calcium, and China's calcium resources are relatively abundant.
But in reality, few electrolytes can simultaneously accommodate or match the highly reducing calcium metal anode and oxygen cathode. Moreover, the discharge products of calcium-oxygen batteries are often relatively inert, making it difficult to charge and discharge multiple times.
The researchers found that the key challenge facing calcium-oxygen batteries with stable charge and discharge at room temperature is that the electrolyte is easily reduced and decomposed by the calcium metal negative electrode, and forms a passivation layer on the electrode surface, causing the negative electrode to fail; while the air positive electrode has a high electrode Electric potential can also easily cause the electrolyte to decompose. People have not been able to find an electrolyte that can match the calcium metal negative electrode and adapt to the high electrode potential air positive electrode.
In order to solve the above challenges, the research team prepared a new electrolyte based on dimethyl sulfoxide/ionic liquid by systematically designing solvents, electrolyte salts and electrolyte ratios.
The newly developed new calcium-oxygen battery mainly consists of three parts: metallic calcium negative electrode, carbon nanotube air positive electrode and organic electrolyte.
The battery achieves a highly reversible two-electron reaction by using a durable ionic liquid new electrolyte to promote the stripping of the calcium metal coating on the negative electrode at room temperature and improve the redox reaction of the air positive electrode. The discharge product of this battery is calcium peroxide.
The researchers say the design of this new calcium-oxygen battery takes into account performance, cost, environmental sustainability requirements and requirements for applications in flexible electronic devices.
Among them, the positive electrode material does not contain expensive precious metal catalysts and uses oxygen in the air as a reactant; the negative electrode material is low in cost and has a high theoretical capacity; the electrolyte exhibits high ionic conductivity and stable electricity at room temperature. Chemical properties improve the overall safety of the battery.
Paper link: https://www.nature.com/articles/s41586-023-06949-x