The reporter learned from the Institute of Modern Physics of the Chinese Academy of Sciences that a scientific research team composed of the institute and cooperative units recently synthesized new nuclides osmium-160 and tungsten-156 for the first time. The relevant results were published in the international academic journal Physical Review Letters on February 15.
The atomic nucleus is a quantum many-body system composed of protons and neutrons. Different numbers of protons and neutrons constitute atomic nuclei with different properties. Scientists call them nuclides. Synthesizing and studying new nuclides is not only of great significance for understanding the structure of matter, but also provides important information for understanding the evolution of the celestial environment, and is an important means of exploring the mysteries of nature.
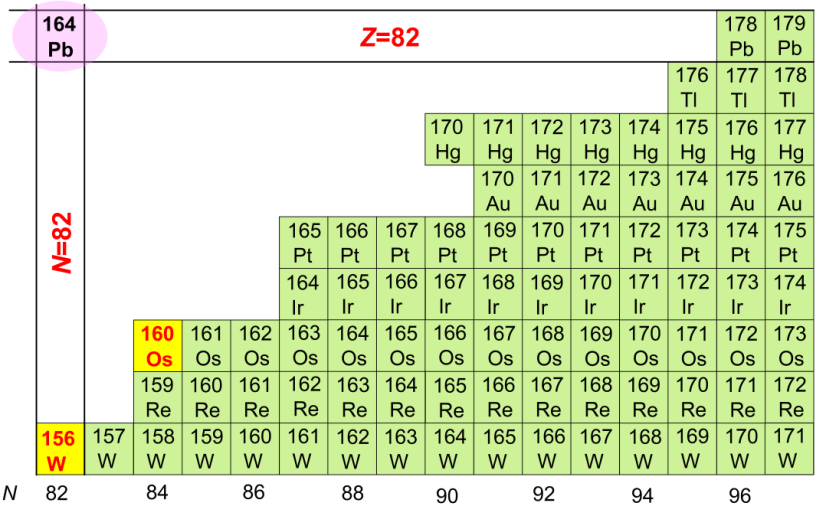
The positions of the new nuclides osmium-160 and tungsten-156 on the nuclide map. Picture/Yang Huabin
The research team relied on the Lanzhou Heavy Ion Accelerator and used the gas-filled recoil nuclear spectrometer SHANS to synthesize new nuclides osmium-160 and tungsten-156 through a fusion evaporation reaction. Osmium-160 (neutron number 84) is alpha radioactive, while tungsten-156 (neutron number 82) is radioactive in beta+ decay. The team measured the alpha decay particle energy and half-life of osmium-160 and the half-life of tungsten-156, among other properties.
Through systematic analysis of new measurement data and existing data, the researchers found that when the atomic number is greater than 68, the preformation probability of alpha particles with neutron numbers 84 and 85 gradually becomes smaller, revealing that the neutron number is 82 The phenomenon that the shell effect is enhanced in neutron-deficient nuclides. Further research suggests that the reason for the enhanced effect is the continuous approach to the potentially stable double phantom nucleus - lead-164 (number of protons is 82, number of neutrons is 82).

Gas Recoil Nuclear Spectrometer SHANS
This study clearly shows for the first time the evolution of a neutron shell with a neutron number of 82 on the neutron-deficient nuclide side, and at the same time, my country's new nuclide research has entered a new nuclear region.
- IewaMtwvhQJEPIG07/23/2025
- OezHagrFvxB07/23/2025
- fQbFrhAAGBM07/22/2025
- sURHpoTo07/22/2025
- kZwfYHmFZRgyiQw07/22/2025