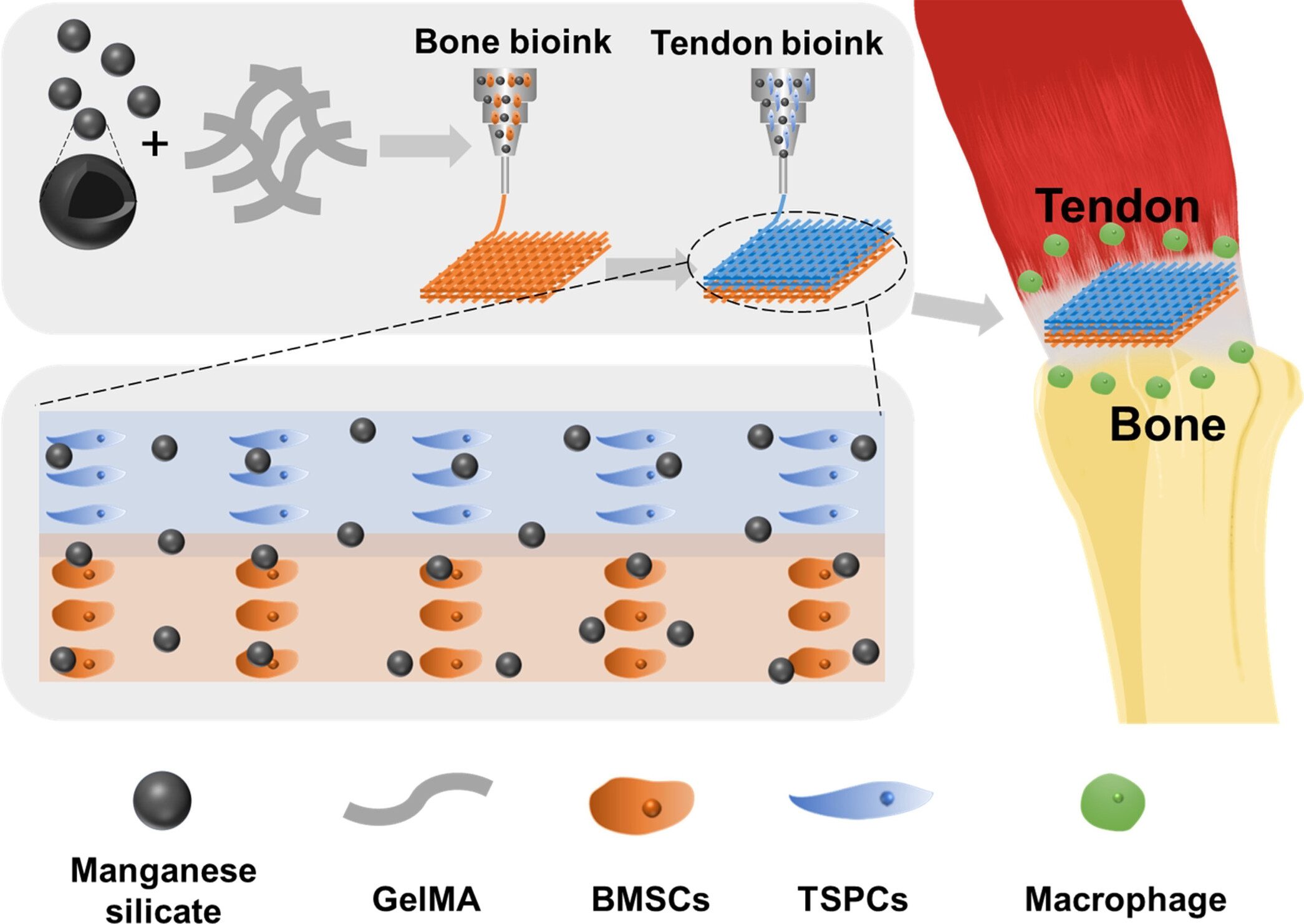
Schematic illustration of immune multicellular scaffold based on manganese silicate (MS) nanoparticles for tendon-bone integrated regeneration.
Recently, a research team led by researcher Wu Chengtie from the Shanghai Institute of Ceramics, Chinese Academy of Sciences, made important progress by using "bioceramics + 3D printing" to create a multi-cell scaffold that can be used for the integrated regeneration of tendons and bones.
The team used multi-cell printing technology to combine manganese silicate (MS) nanoparticles with tendon/bone-related cells to design and construct the above-mentioned multi-cell scaffold with immune regulatory functions.
Among them, tendon stem/progenitor cells (TSPCs) and bone marrow mesenchymal stem cells (BMSCs) are distributed in the scaffold in a hierarchical manner, realizing the simulation of the tendon-bone interface and exhibiting bidirectional differentiation activities into osteogenesis and tendonogenesis.
Experiments on rabbits and rats show that the multi-cell scaffold simultaneously achieves immune regulation, interface microstructure regeneration and motor function recovery. It can also stably release manganese ions to stimulate macrophages to secrete PGE2, thereby enhancing the specific differentiation of multi-cells.
The research results were published online on March 8 in the academic journal Science Advances. The article was titled "Immunomodulatory Multicellular Scaffolds for Tendon-to-Bone Regeneration" and an invention patent was applied for. The first authors of the above paper are master student Du Lin and doctoral student Wu Jinfu of Shanghai Institute of Ceramics, and the corresponding author is researcher Wu Chengtie.
The unique structure of the tendon-bone interface can effectively relieve stress concentration and plays an important role in human movement function.
When injury occurs, due to its complex physiological structure and poor regeneration ability, clinical surgical treatment often leads to the formation of scar tissue at the interface, resulting in a high probability of re-injury.
Traditional biomaterials tend to enhance biological functions directly related to tendon-bone, such as osteogenic differentiation or tenogenic differentiation, but the three-dimensional microenvironment of the injury site, especially the inflammatory response triggered by immune cells in the body.
The above-mentioned newly published immune multicellular scaffolds provide a new strategy for integrated regeneration of soft and hard tissue interfaces.



Whoa quite a lot of good tips! casino en ligne Nicely put. With thanks! casino en ligne Fantastic data. Cheers! casino en ligne Very good info, Many thanks. casino en ligne Excellent postings Many thanks. casino en ligne With thanks, I appreciate this. casino en ligne Kudos! Very good stuff. casino en ligne Regards, Lots of posts! casino en ligne With thanks, I value this! casino en ligne You revealed this well. casino en ligne